Orally disintegrating tablet
A technology of orally disintegrating tablets and additives, which is applied in the direction of pill delivery, digestive system, organic active ingredients, etc., can solve the problems of fine particle breakage, reduce bitterness, acid resistance and masking effect, reduce the frequency of dosing and maintain the effectiveness of treatment Concentration, the effect of ensuring the effectiveness of the treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0149] The production method of wet compression tableting is preferably the method described in JP-A-5-271054 and the like. It can also be prepared by wetting followed by drying. The method is preferably the method described in JP-A-9-48726, JP-A-8-291051 and the like. That is, it is effective in increasing hardness by wetting before and after tableting and then drying.
[0150] Raw material powders and granules can be punched at room temperature, or can be heated and compressed at a temperature not lower than room temperature (about 25°C to about 40°C). In this specification, the "room temperature" refers to the room temperature for tableting for ordinary tablet preparation, which is generally about 20°C to about 25°C.
[0151] The "drying" can be carried out by any method commonly used for drying preparations, such as vacuum drying, fluidized bed drying and the like.
[0152] The tablet (I) and tablet (II) of the present invention may also optionally contain additives as ...
Embodiment
[0181] The present invention is explained in more detail below by referring to Preparation Examples, Reference Examples, Examples, Comparative Examples and Experimental Examples, which do not limit the present invention.
[0182] Additives (for example, mannitol, sucralose) used in the following preparation examples, reference examples, examples and comparative examples are the corresponding products of the 15th edition of the Japanese Pharmacopoeia or Japanese pharmaceutical excipients 2003. In the following preparation examples and reference examples, compound X is (R)-2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl] Sulfinyl]-1H-benzimidazole. Properties of fine particles, granules and preparations obtained in Preparation Examples, Reference Examples, Examples and Comparative Examples were determined by the following test methods.
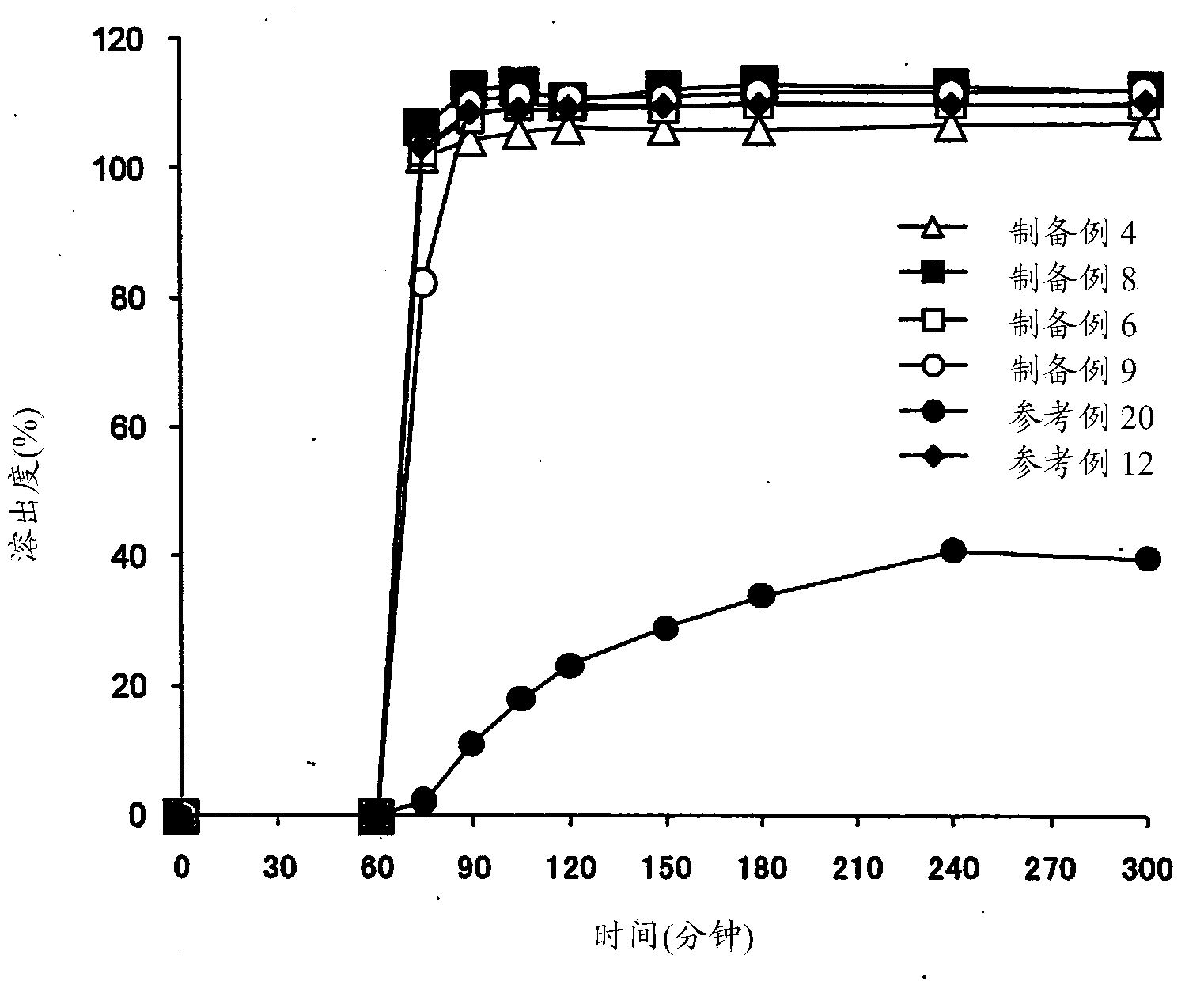
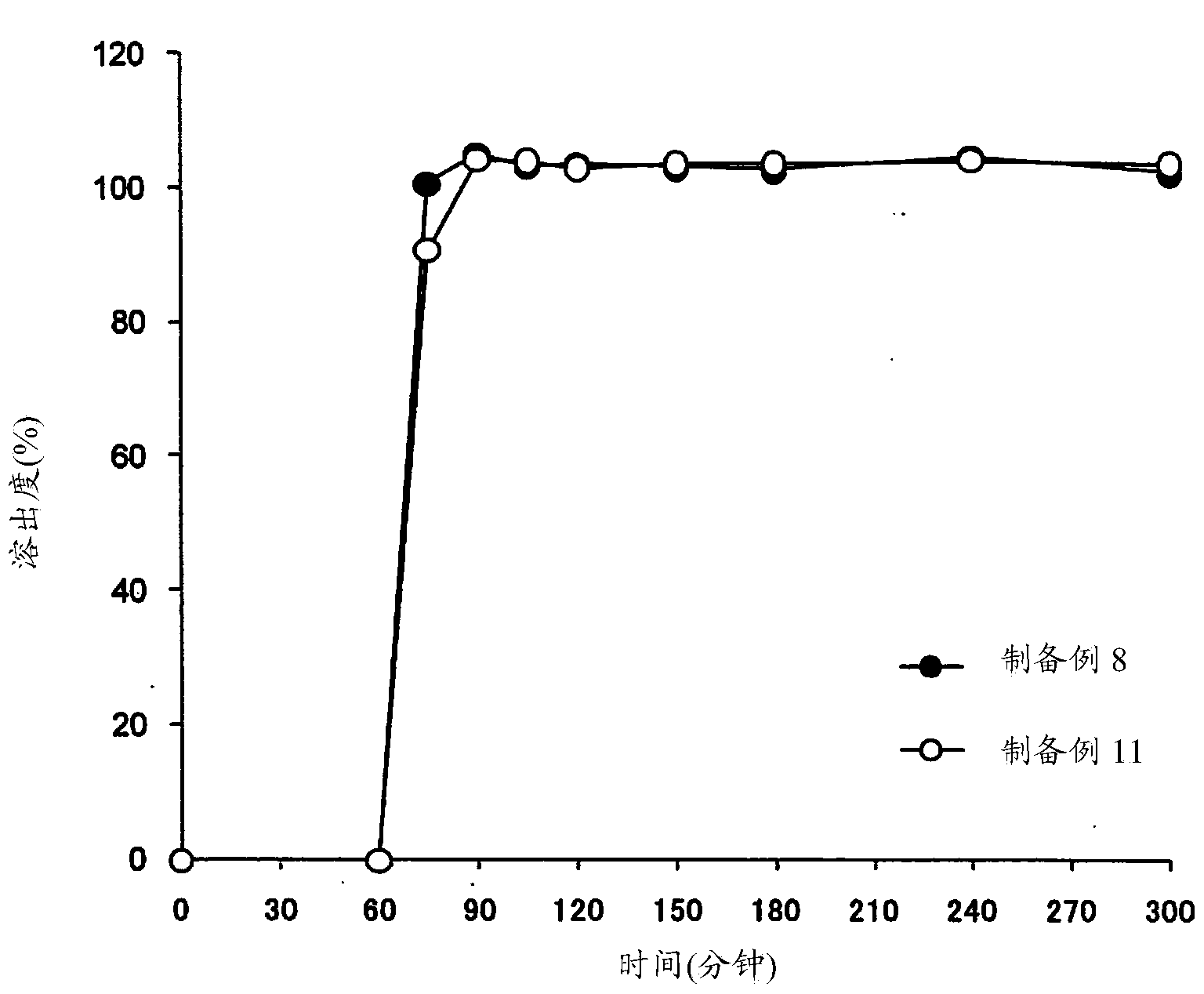
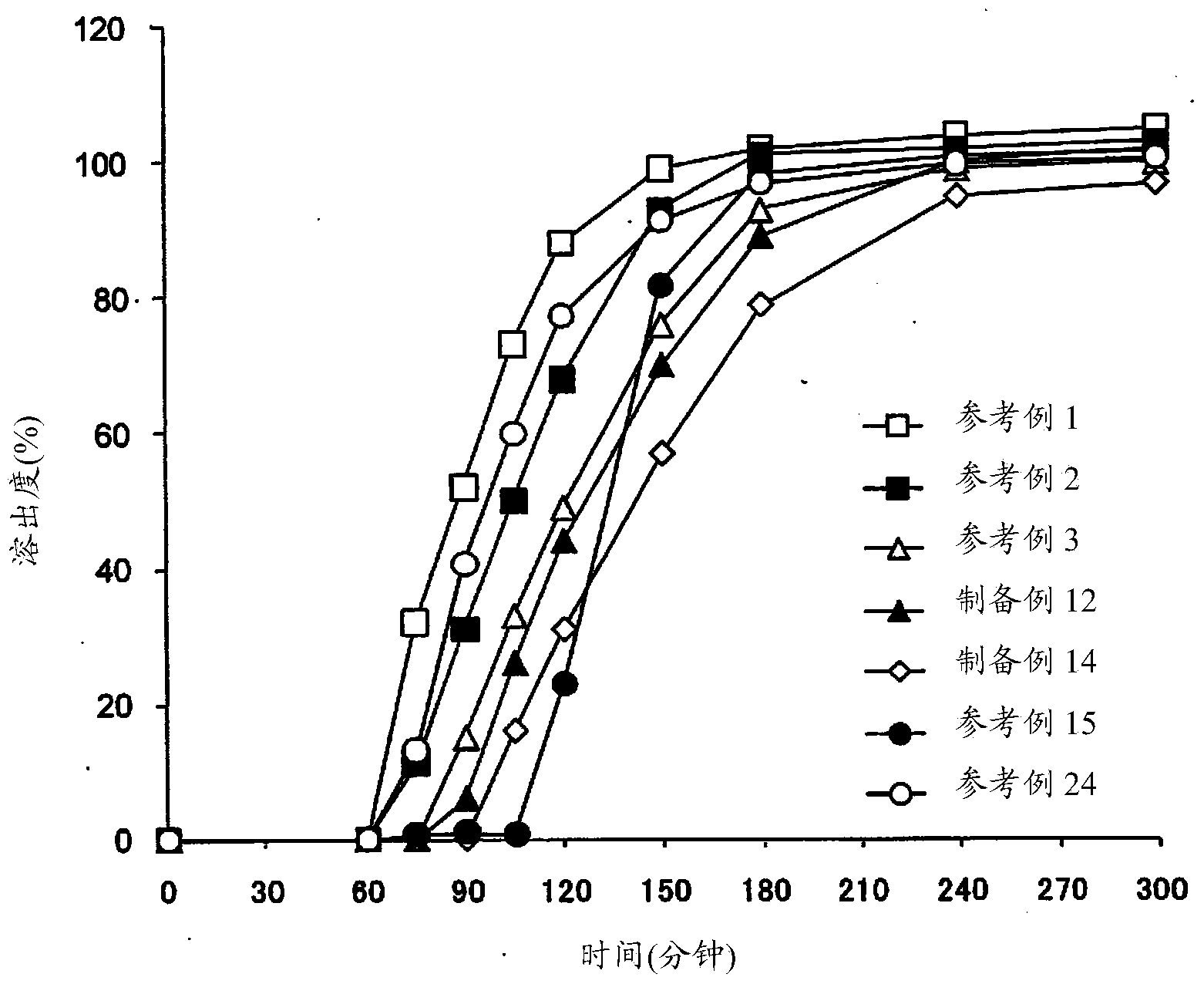
[0183] (1) Dissolution test
[0184] Dissolution testing was performed by either of the following methods using the basket method (U...
preparation example 1
[0198] Preparation of fine granules containing pharmaceutically active ingredients
[0199] Hydroxypropylcellulose (360 g) was dissolved in purified water (4680 g), and low-substituted hydroxypropylcellulose (L-HPC-32, 180 g) and magnesium carbonate (360 g) were dispersed in the solution. Compound X (1080 g) was uniformly dispersed in the resulting dispersion to obtain a coating solution. Lactose / crystalline cellulose spheres (Nonpareil 105T, 900 g) were coated with a predetermined amount (5550 g) of compound X-containing coating solution (6660 g) by using an oscillating fluidized bed coater (MP-10TOKU-2 type, POWREX Corporation production) to carry out. The coating conditions are: the inlet air temperature is about 85°C, the spray air pressure is about 0.25MPa, the spray air volume is about 80Nl / min, and the inlet air volume is about 0.7m 3 / min, the rotor rotation rate is about 500rpm, the spray rate is about 15g / min, and the spray position is at the bottom.
[0200] [Com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com