Patents
Literature
45 results about "Schizosaccharomyce pombe" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
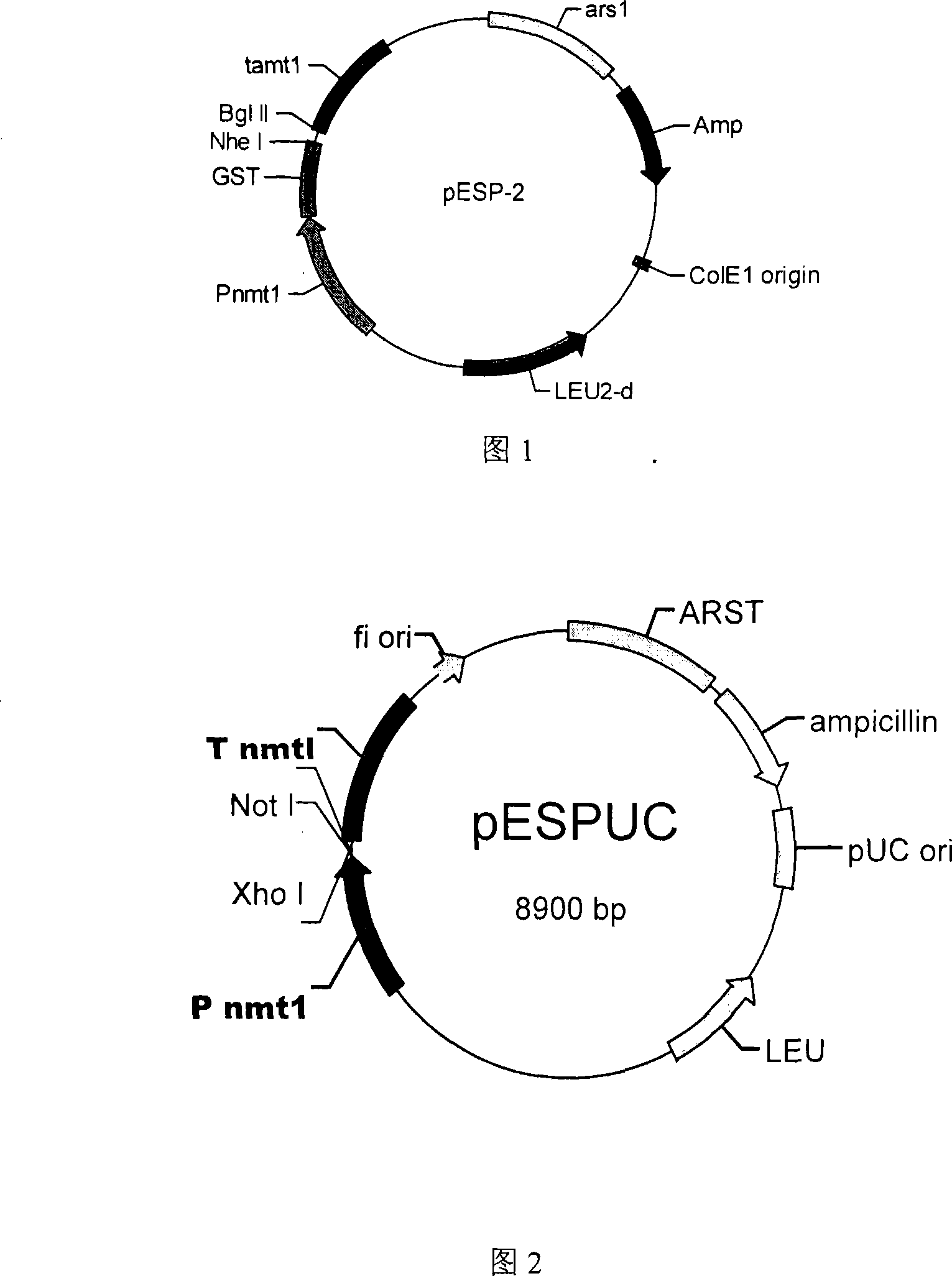
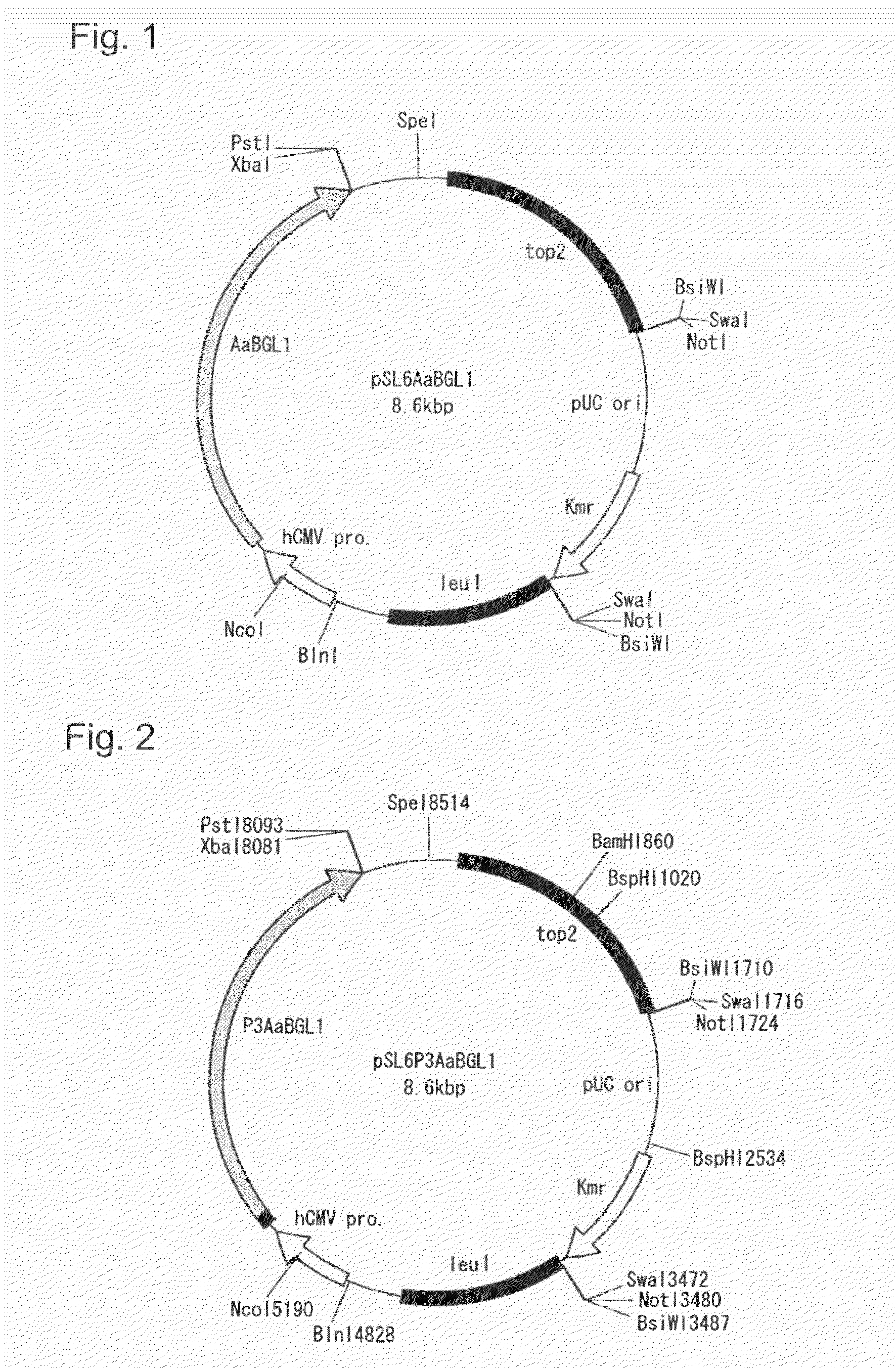
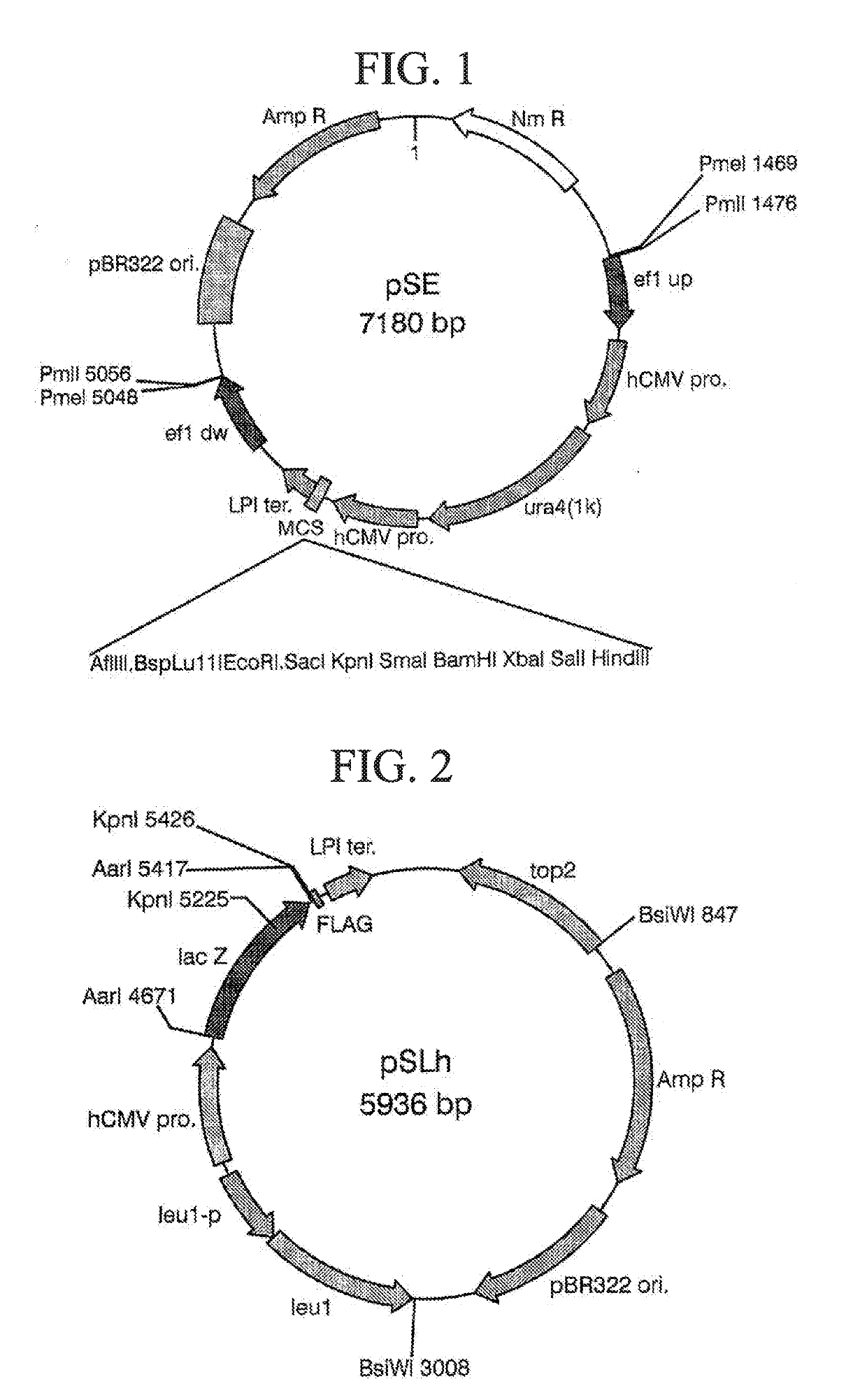
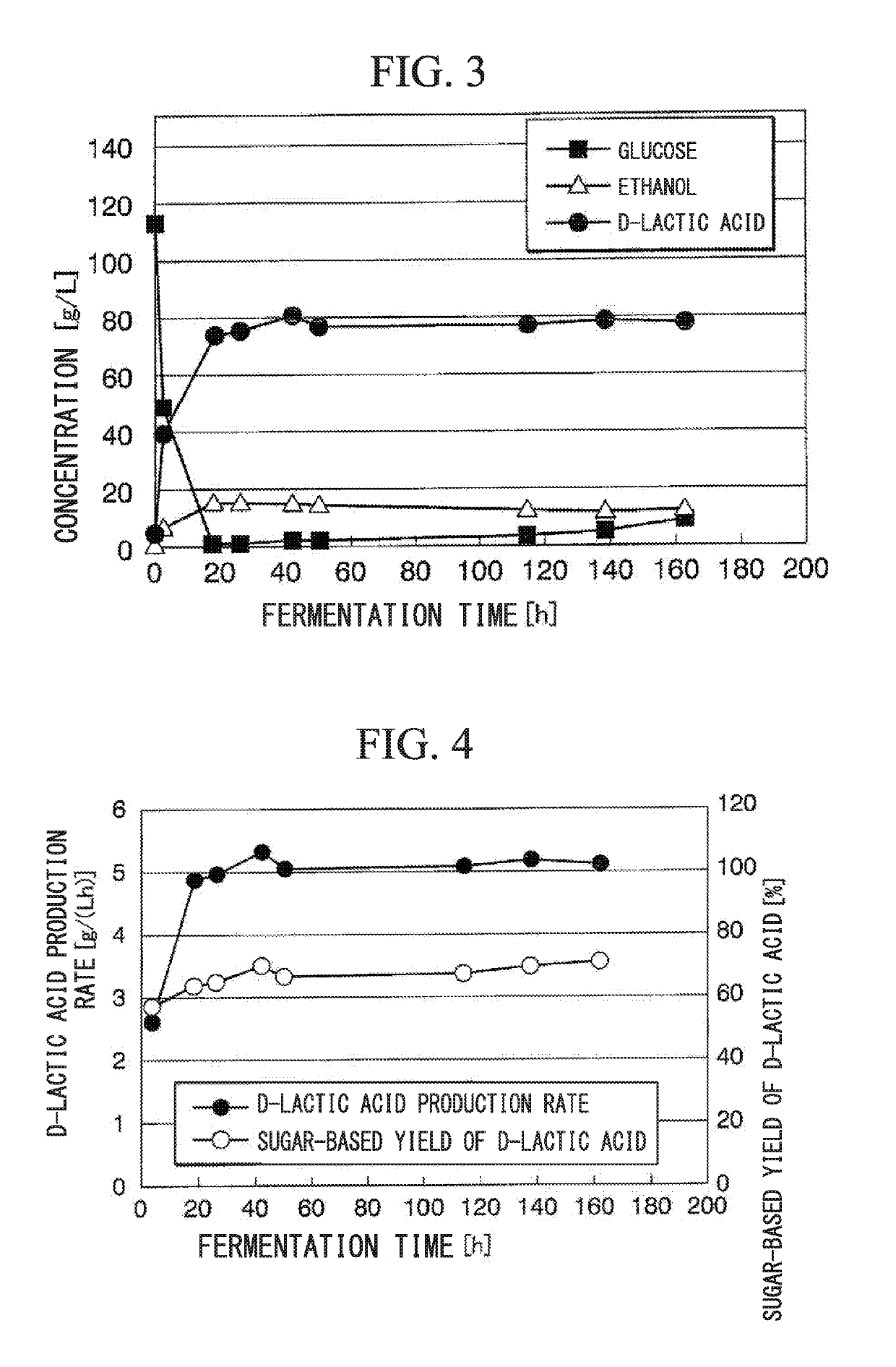
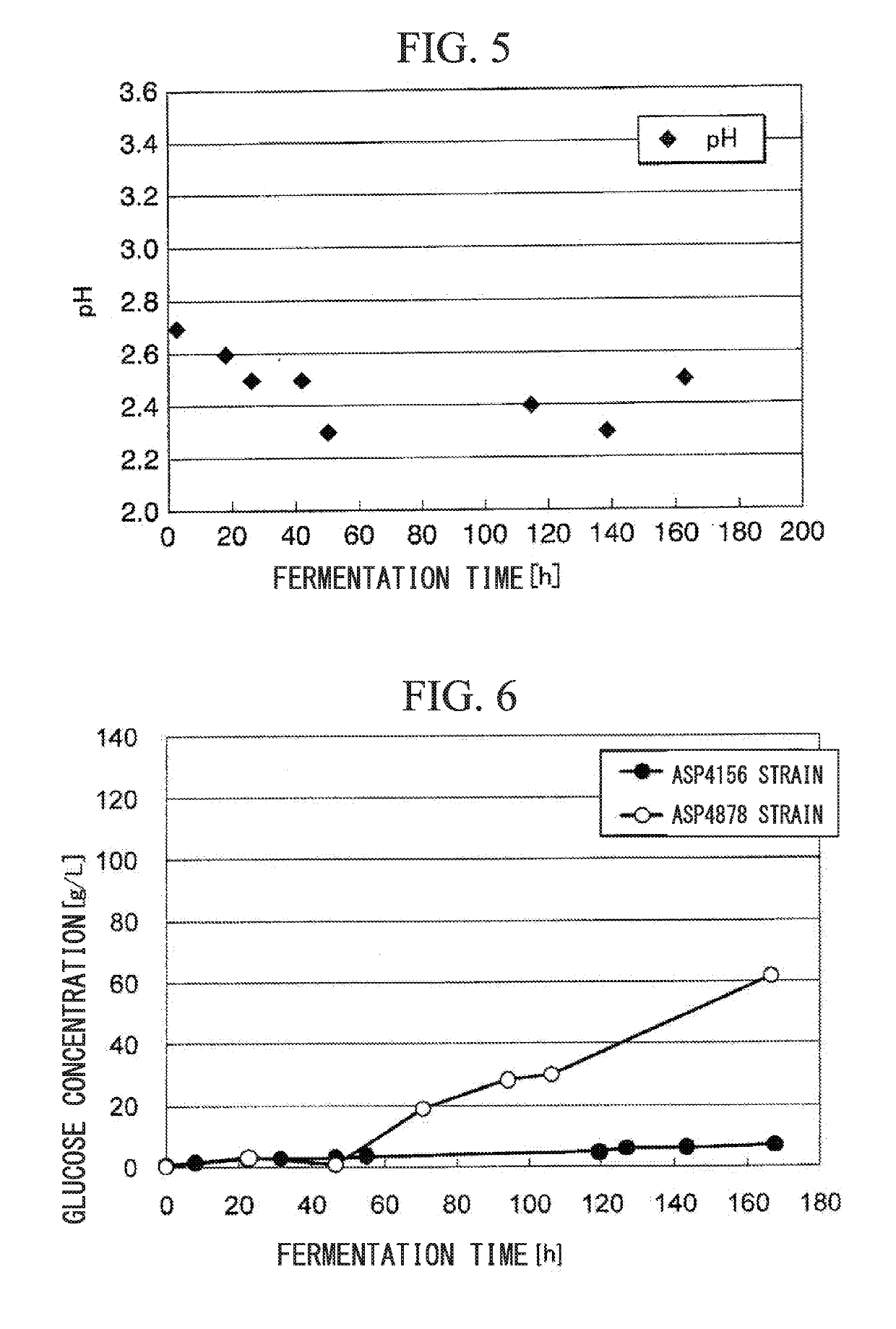
Transformant and process for production thereof, and process for production of lactic acid
The present invention relates to a transformant, containing a lactate dehydrogenase gene which is introduced into Schizosaccharomyces pombe as a host, in which a part of a gene cluster encoding a pyruvate decarboxylase in the Schizosaccharomyces pombe host is deleted or inactivated.
Owner:ASAHI GLASS CO LTD
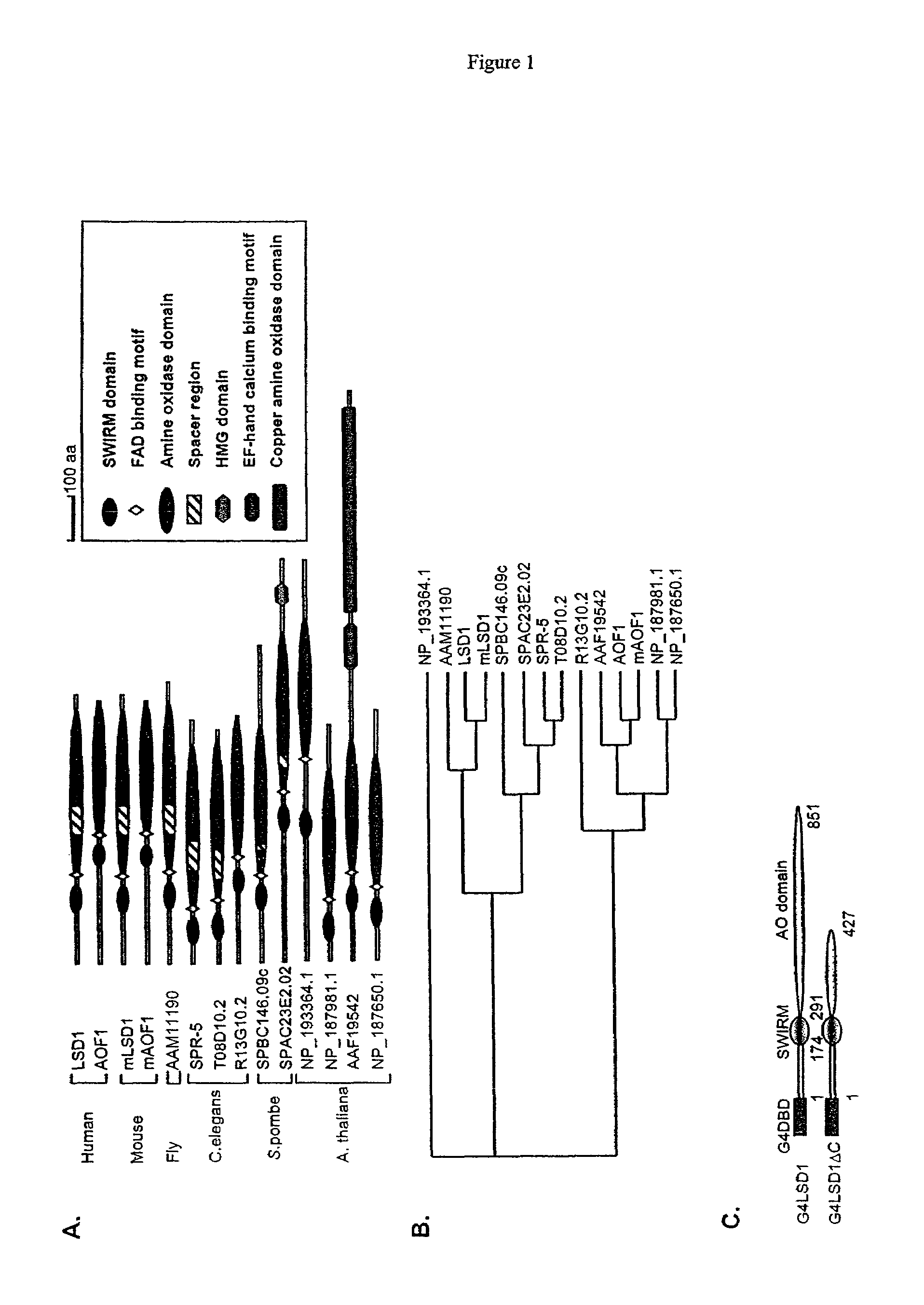
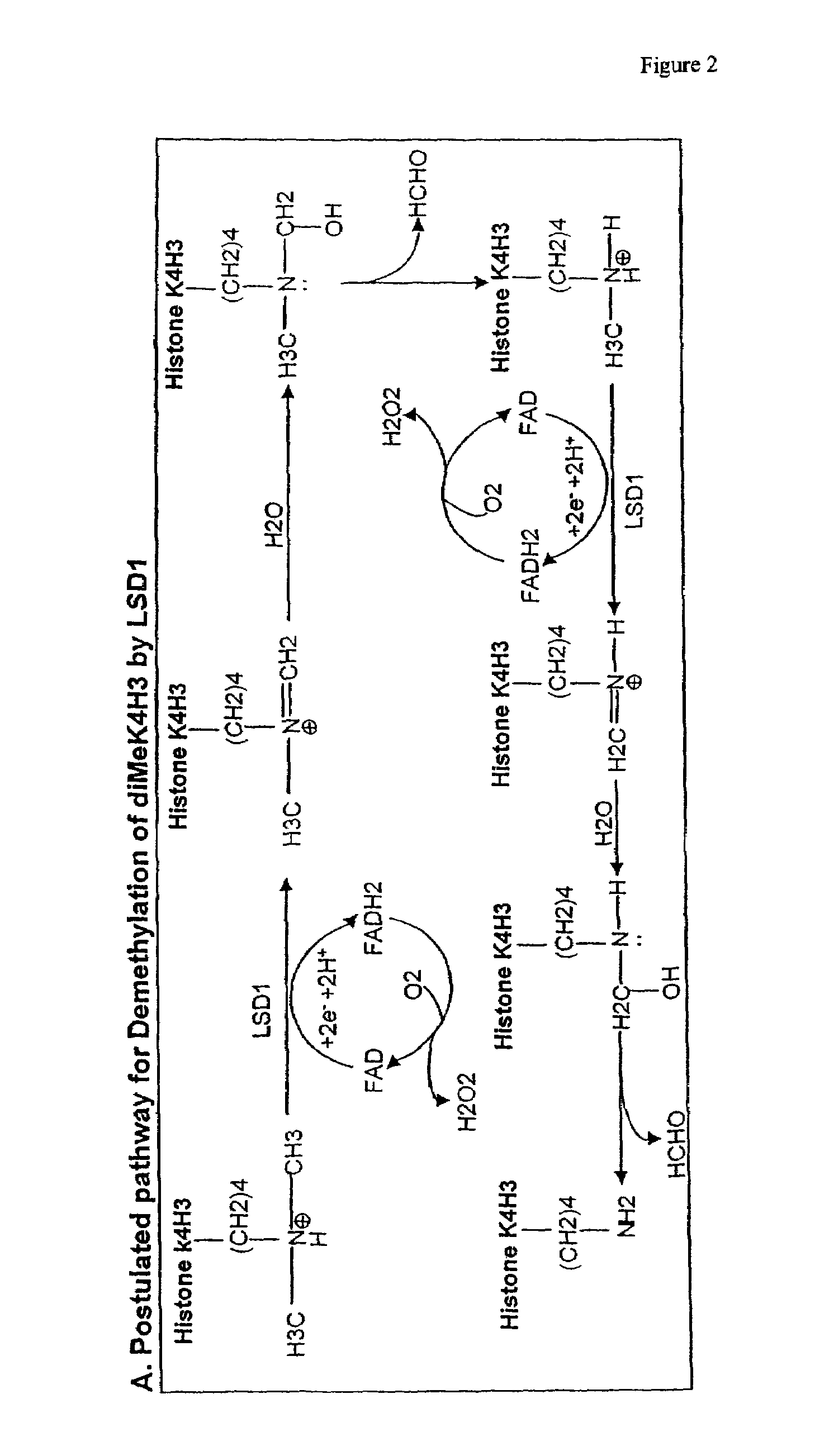
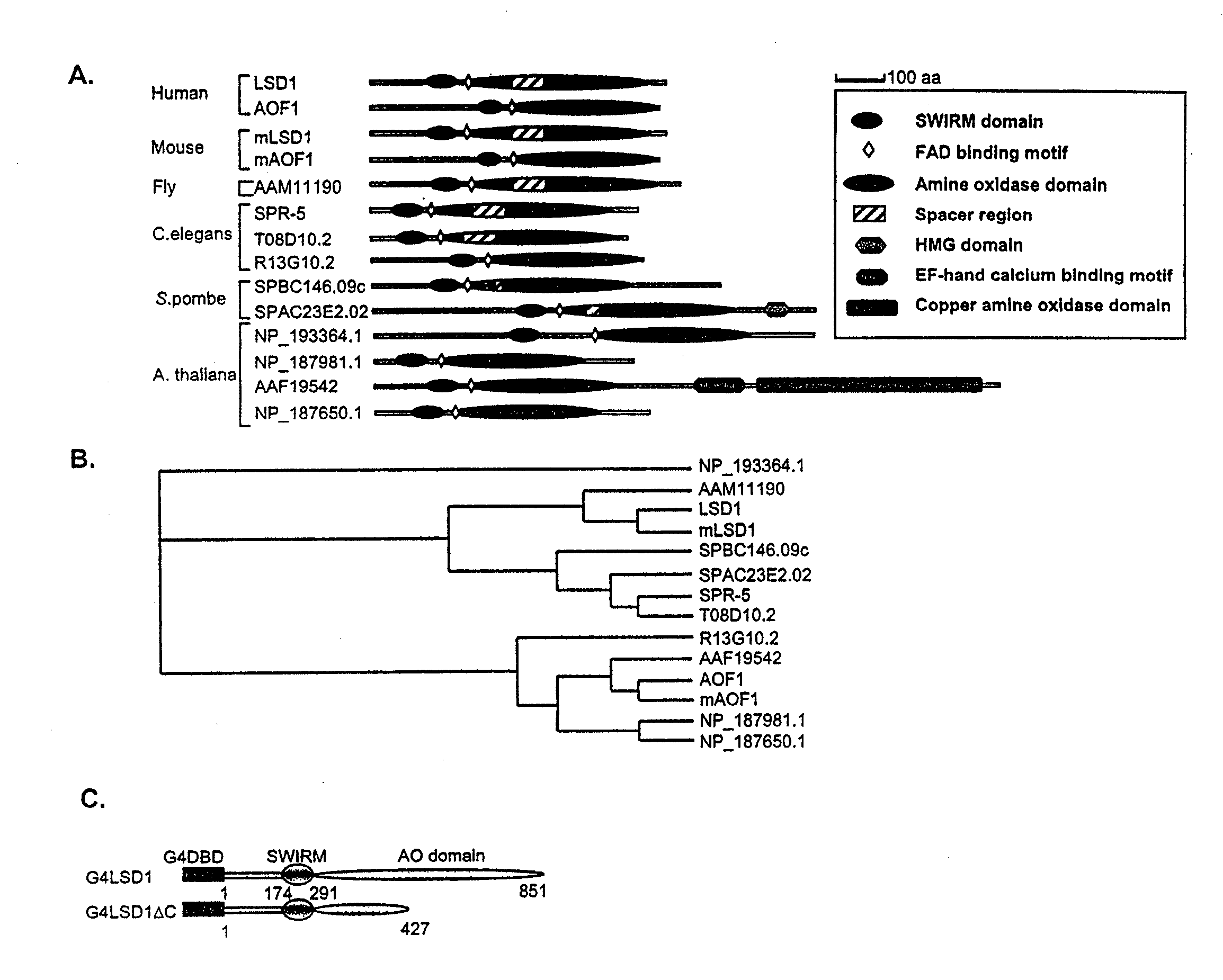
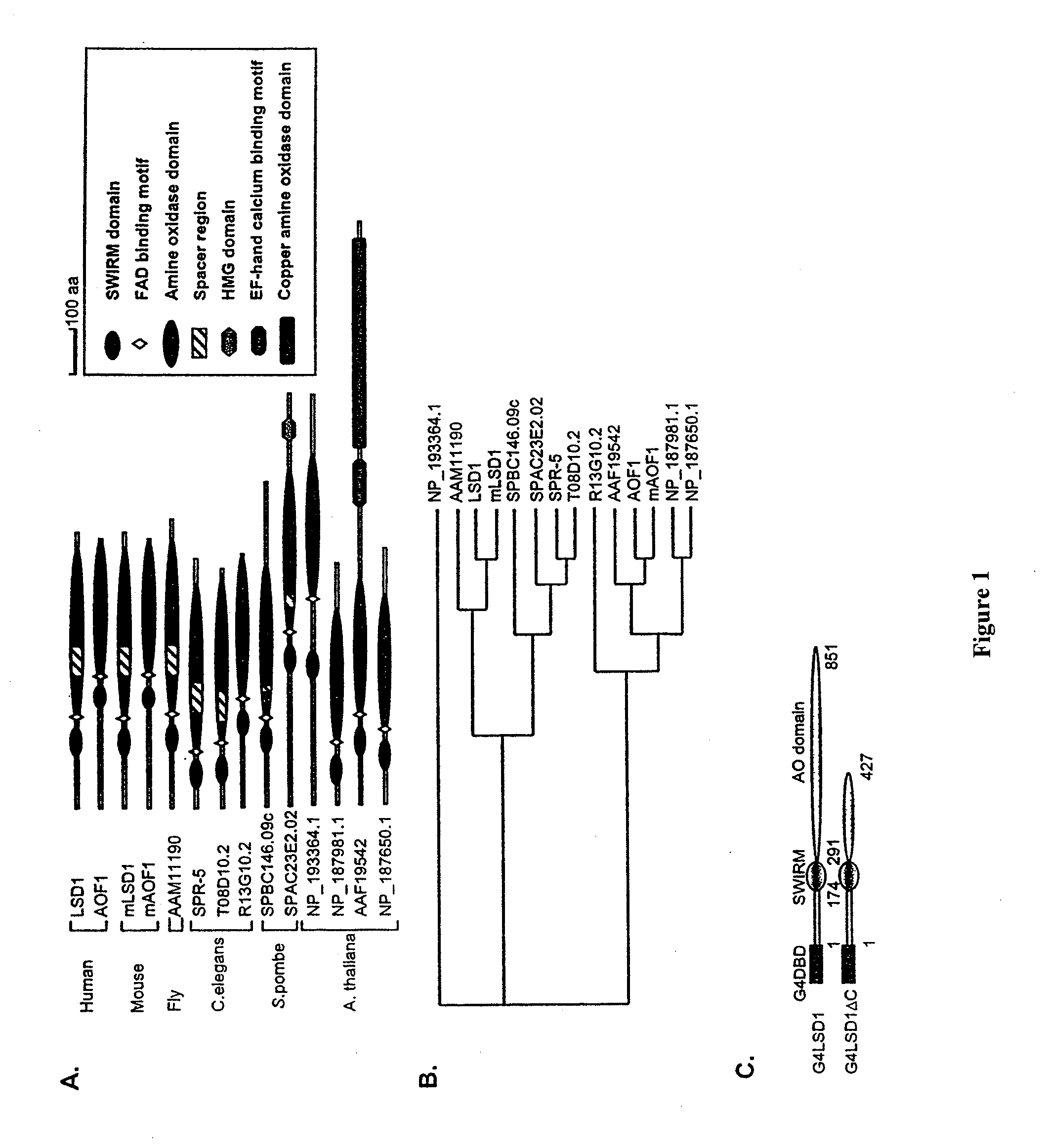
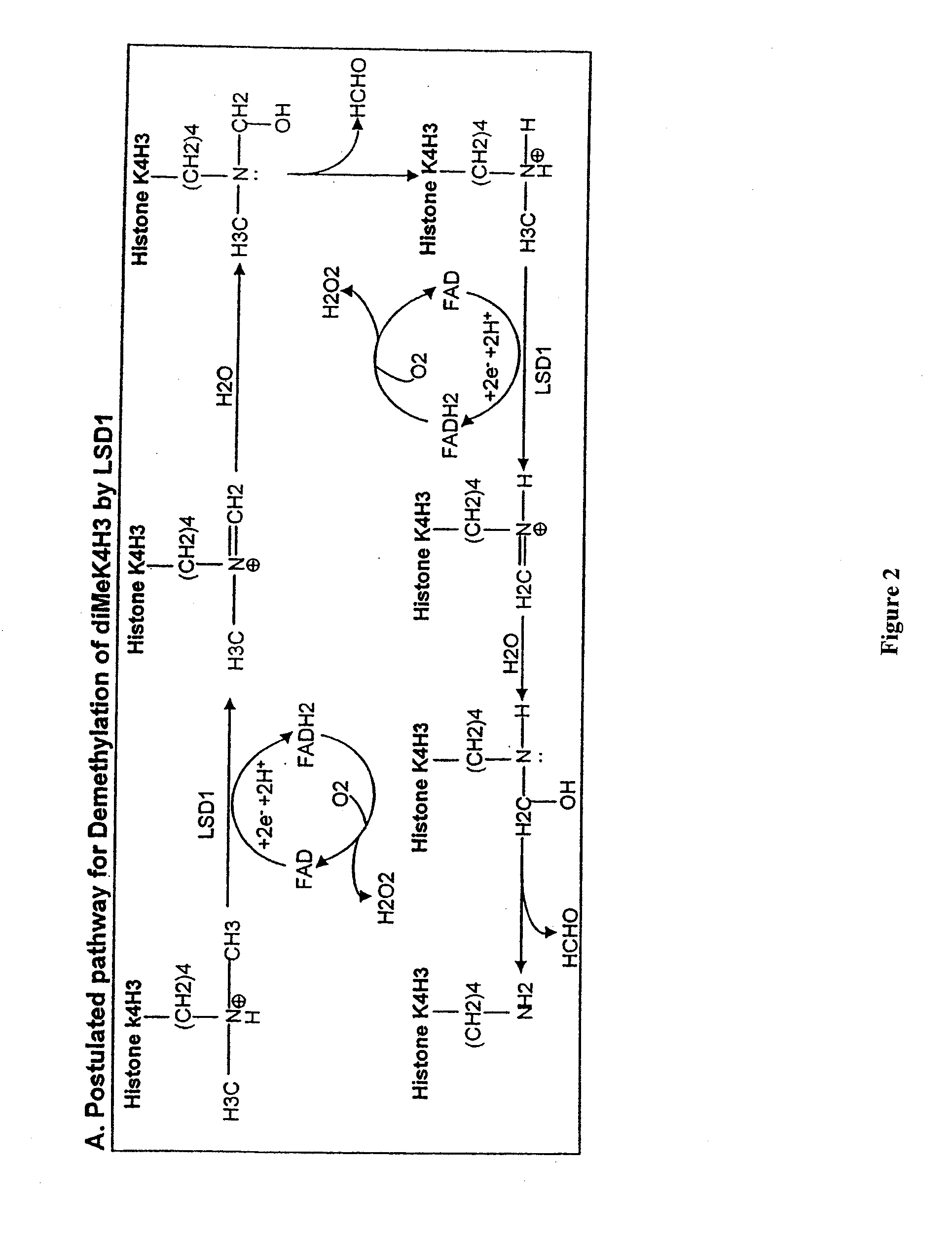
Histone demethylation mediated by the nuclear amine oxidase homolog LSD1
LSD1, a homolog of nuclear amine oxidases, functions as a histone demethylase and transcriptional co-repressor. LSD1 specifically demethylates histone H3 lysine 4, which is linked to active transcription. Lysine demethylation occurs via an oxidation reaction that generates formaldehyde. Importantly, RNAi inhibition of LSD1 causes an increase in H3 lysine 4 methylation and concomitant de-repression of target genes, suggesting that LSD1 represses transcription via histone demethylation. The results thus identify a histone demethylase conserved from S. pombe to human and reveal dynamic regulation of histone methylation by both histone methylases and demethylases.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Composite microbial agent for treating domestic garbage and method for treating domestic garbage by using composite microbial agent
InactiveCN106978370AIncrease functional diversityImprove applicabilityFungiBacteriaPseudomonas putidaMicrobial agent
The invention relates to a composite microbial agent for treating domestic garbage and a method for treating the domestic garbage by using the composite microbial agent. The composite microbial agent for treating the domestic garbage is prepared from the following components: brevendimonas diminuta, pseudomonas stutzeri, pseudomonas maltophilia, pseudomonas fluorescens, pseudomonas putida, candida utilis, candida lipolytica, schizosaccharomyces pombe, schizosaccharomyces octosporus, bacillus megatherium, bacillus subtilis, bacillus cereus, alcaligenes faecalis, clostridium beijerinckii, achromobacter denitrificans, nocardia coralline, and the like, wherein the parts by weight of the brevendimonas diminuta are 1-5, the parts by weight of the pseudomonas stutzeri are 1-5, the parts by weight of the pseudomonas maltophilia are 1-5, the parts by weight of the pseudomonas fluorescens are 1-5, the parts by weight of the pseudomonas putida are 1-5, the parts by weight of the candida utilis are 1-5, and the parts by weight of the candida lipolytica are 1-5.
Owner:哈尔滨明慧生物技术开发有限公司
Biological acid reduction method for red bilberry wine
ActiveCN101440340AImprove taste qualityAvoid quality impactAlcoholic beverage preparationYeastFruit wine
The invention discloses a method for biological deacidification of blueberry wine, which comprises the following steps: preparation of blueberry juice, fermentation, separation, post-fermentation, clarification and bottling. The method adopts the prior fruit wine brewing process to prepare raw blueberry wine, and then uses pombe fission yeast to ferment so as to perform the biological deacidification. Compared with the prior method for brewing the blueberry wine, the method has the following advantages: (1) improving the mouthfeel quality of the blueberry wine; (2) leading the active components from blueberry berry to the blueberry wine; (3) avoiding the influence of chemical deacidification on the quality of the blueberry wine; and (4) guaranteeing that the brewed wine is full-juice blueberry wine.
Owner:吉林森工集团泉阳泉饮品有限公司
Multi-microbe solid fermentation ethanol and acetic acid production key microbe quantitative analysis method
ActiveCN107523625AStable amplificationAmplification results are clearMicrobiological testing/measurementMicroorganism based processesAcetic acidMicroorganism
The invention relates to the technical field of liquor production, particularly to a multi-microbe solid fermentation ethanol and acetic acid production key microbe quantitative analysis method. The method comprises performing quantitative PCR (polymerase chain reaction) analysis on HIS3 genes of saccharomyces cerevisiae, AHD1 genes of schizosaccharomyces pombe and 16S rRNA (ribonucleic acid) fragments of lactobacillus to analyze the change tendency of the saccharomyces cerevisiae, the schizosaccharomyces pombe and the lactobacillus in liquor yeasts and fermented grains and accordingly to predict the yield of ethanol. The multi-microbe solid fermentation ethanol and acetic acid production key microbe quantitative analysis method has the advantages of being high in detecting speed and sensitivity, good in accuracy and specificity and free from cultivation.
Owner:KWEICHOW MOUTAI COMPANY
Method for producing edible alcohol by fermenting thick mash of defective red dates at high temperature
ActiveCN104046542AComply with hygienic standardsMicroorganism based processesAlcoholic beverage preparationBiotechnologyAlcohol
The invention discloses a method for producing edible alcohol by fermenting thick mash of defective red dates at a high temperature. According to the advantages of high sugar content of defective red dates, defective red dates are taken as raw materials, by virtue of high-temperature alcohol-resistant Schizosaccharomyces pombe, and the thick mash of red dates is subjected to high-temperature alcoholic fermentation to obtain red dates edible alcohol dates. According to the method, the conversion rate from carbohydrates in red dates to ethanol reaches above 80%, and the produced red dates edible alcohol is in line with hygienic standards of national edible alcohol.
Owner:SHAANXI NORMAL UNIV
Schizosaccharomyces pombe engineering strain having cellulase activity and constructing method thereof
InactiveCN101157898ANormal biological activityLow costFungiOther foreign material introduction processesBiotechnologyProkaryotic expression
The invention discloses an engineering bacteria of fission yeast schizosaccharomyces pombe which has cellulase activity and a construction method thereof. A constructed expression carrier of the fission yeast schizosaccharomyces pombe which contains cellulase genes is led into the yeast schizosaccharomyces pombe and the fission yeast schizosaccharomyces pombe provided by the invention is obtained. The construction method of engineering bacteria of fission yeast schizosaccharomyces pombe is that the fission yeast schizosaccharomyces pombe which contains cellulase genes is constructed and then led into the fission yeast schizosaccharomyces pombe to gain a recombinant yeast transformant, and then the recombinant yeast cultured to lead cellulase genes to express the carrier. The invention makes up the shortcomings of the traditional preparation method of cellulase, a prokaryotic expression system, and the expression system of saccharomyces cerevisiae and pichia. The invention is applied to the production of industrial cellulase and has advantages of high activity of cellulase, short growth cycle, and low cost of extraction.
Owner:JILIN AGRICULTURAL UNIV
Traditional Chinese medicinal preparation for treating damp turbidity and spleen stagnation type hyperlipidemia
ActiveCN103585523AGood curative effectSmooth stoolMetabolism disorderPlant ingredientsCorn silkAdemetionine
A traditional Chinese medicinal preparation for treating damp turbidity and spleen stagnation type hyperlipidemia is prepared by using malt, millet sprout, rice grain sprout, Gynostemma pentaphylla, lotus leaf, corn silk, Chinese yam, dried orange peel, cassia seed, hawthorn fruit, Mythic Fungus, wolfberry fruit, Poria cocos, white hyacinth bean, lotus seed, black soybean, jujube, Coriolus versicolor(L.ex Fr.)Quel., dahurian angelica root, coix seed, Ligustri Lucidi Fructus and Semen Phaseoli. A preparation method of the traditional Chinese medicinal preparation comprises the following steps: decocting the above raw materials with water, cooling the obtained filtrate to room temperature, and inoculating Saccharomyces cerevisiae or Sac.carlsbergensis Hansen or Schizosaccharomyces pombe or Nematspora gossypii, wherein the amount of the above inoculated bacterium accounts for 1-5% of the volume of the water extract filtrate; fermenting at 20-32DEG C for 30-48h to obtain a suspension fermentation liquid, concentrating to obtain an extract paste, and drying at 80-100DEG C; and grinding the dried extract paste to fine powder having a particle size of 150mum or less to obtain an inactivated thallium and metabolite fine powder, detecting the protein content reaching 40% or more, and filling to a capsule. The clinic cure rate, the significant efficiency, the effective rate and the total effective rate of the preparation are 32.90%, 23.81%, 32.14% and 92.86% respectively.
Owner:ZIBO DEV ZONE YADA PHARMA
Environment-friendly restoring agent for heavy metals in soil
InactiveCN108329083APrevent compactionRestore activitySuperphosphatesAgriculture tools and machinesSodium BentoniteSchizosaccharomyces pombe
The invention discloses an environmental-friendly restoring agent for heavy metals in soil. The environmental-friendly restoring agent is prepared from the following materials by weight: 15-28 parts of carboxymethyl chitosan, 17-31 parts of polyacrylamide,7-19 parts of polyaluminum chloride, 20-39 parts of polyaluminum ferric chloride, 14-31 parts of bentonite, 17-28 parts of sepiolite, 7-11 partsof calcium magnesium phosphate fertilizer, 28-33 parts of calcium superphosphate, 20-31 parts of magnesium sulfate, 15-27 parts of sodium silicate, 13-23 parts of boric acid, 21-31 parts of borax, 28-37 parts of zinc sulfate, 11-21 parts of amino acids, 9-14 parts of humic acid, 13-18 parts of a compound enzyme preparation, 4-6 parts of slash pine needles, 4-6 parts of Pseudomonas fluorescens powder, 7-11 parts of halotolerant yeast powder and 10-15 parts of Schizosaccharomyces pombe powder. The environmental-friendly restoring agent for heavy metals in soil can supplement a large amount of organic matters and nitrogen-phosphorus-potassium nutrient sources, reduce the activity of heavy metals in soil, improve the physical and chemical properties of soil, lower soil acidity, and achieve the purpose of restoring soil activity and fertility.
Owner:HENAN INST OF SCI & TECH
Preparation and quick screening method of yeast fusant
The invention provides a preparation and quick screening method of yeast fusant. In order to realize mass production of coenzyme Q10, a method of protoplast fusion is utilized to fuse schizosaccharomyces pombe and hensenula polymorpha and combined with a method for efficiently screening target yeast fusant to quickly and efficiently obtain target yeast fusant having excellent characteristics of two yeast strains, and the target yeast fusant has the advantages of high growing speed, resistance to high temperature and capability of efficiently producing the coenzyme Q10. According to the yeast fusant screening method, growing performance including growing speed, resistance to high temperature and target metabolite of thalli is taken as a screening marker; specifically, temperature tolerance and a culture medium containing vitamin K3 which is a structural analog of the coenzyme Q10 are taken as screening strategies, and the target fusant can be directly screened in a high-throughput manner. The preparation and quick screening method is a yeast improving method which is simple, quick, efficient, economical, practical and safe.
Owner:SHAANXI UNIV OF SCI & TECH
Method for preparing coenzyme Q10 by microbial transformation under condition of supercritical CO2
InactiveCN101591685AImprove solubilityEasy to synthesizeFungiMicroorganism based processesSolubilityMicrobial transformation
The invention discloses a method for preparing coenzyme Q10. The method of the invention comprises the following steps: switching-in schizosaccharomyces pombe to culture medium containing solanesol, carrying out fermentation in the supercritical CO2 environment with temperature ranging from 25 DEG C to 35 DEG C and pressure ranging from 5MPa to 20 MPa, thus obtaining the coenzyme Q10.The method of the invention makes full use of dissolution characteristics of the supercritical CO2 to increase solubility of the solanesol, thus effectively reducing product inhibiting effect in the process of conversion, providing a high anaerobic environment for yeast, changing respiratory chain oxygen supply mode of microorganism, and promoting synthesis of the coenzyme Q10; in addition, in the method of the invention, the solanesol is added as substrate of the coenzyme Q10, thus improving speed and output of the synthesis of the coenzyme Q10.Experiments indicate that the output of the coenzyme Q10 prepared by the method of the invention is increased by 60-162% compared with the output thereof prepared by ordinary fermentation methods.
Owner:CHINA AGRI UNIV
Acid-resistant saccharomyces cerevisiae and application thereof in preparation of organic acid
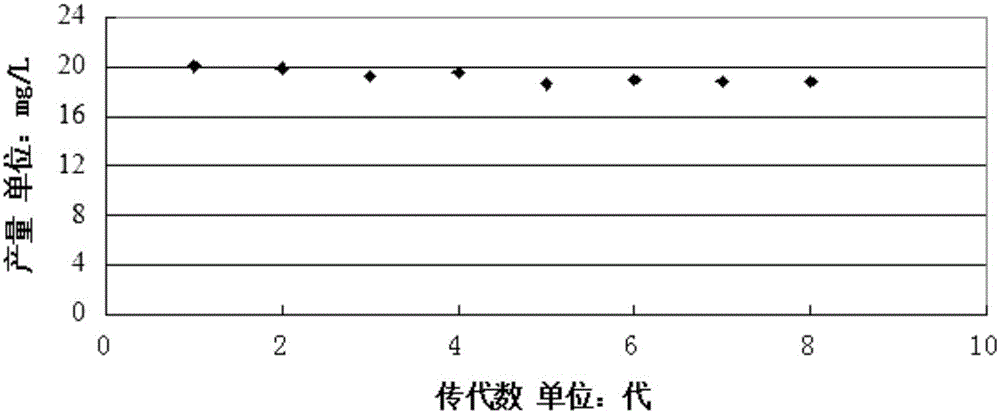
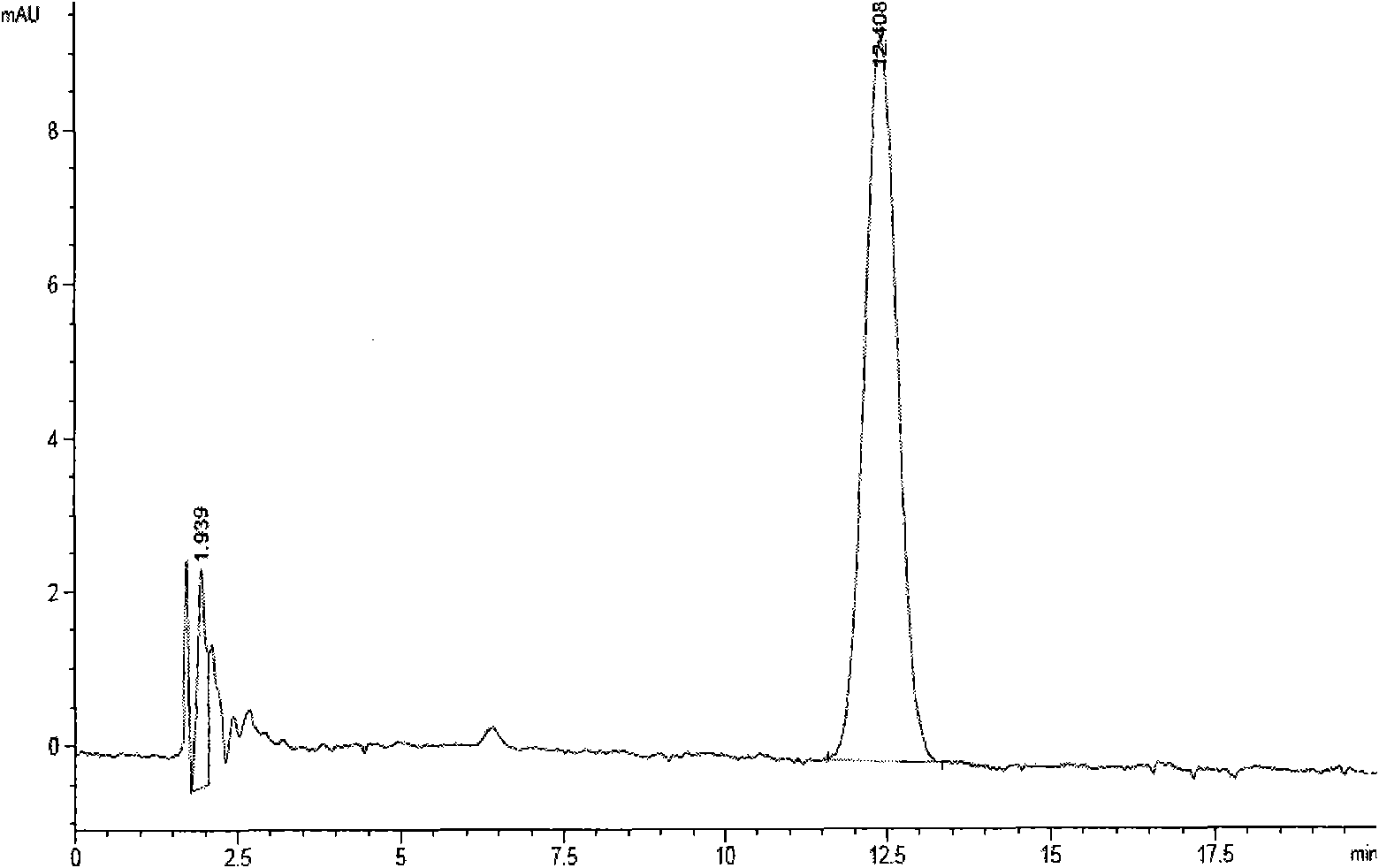
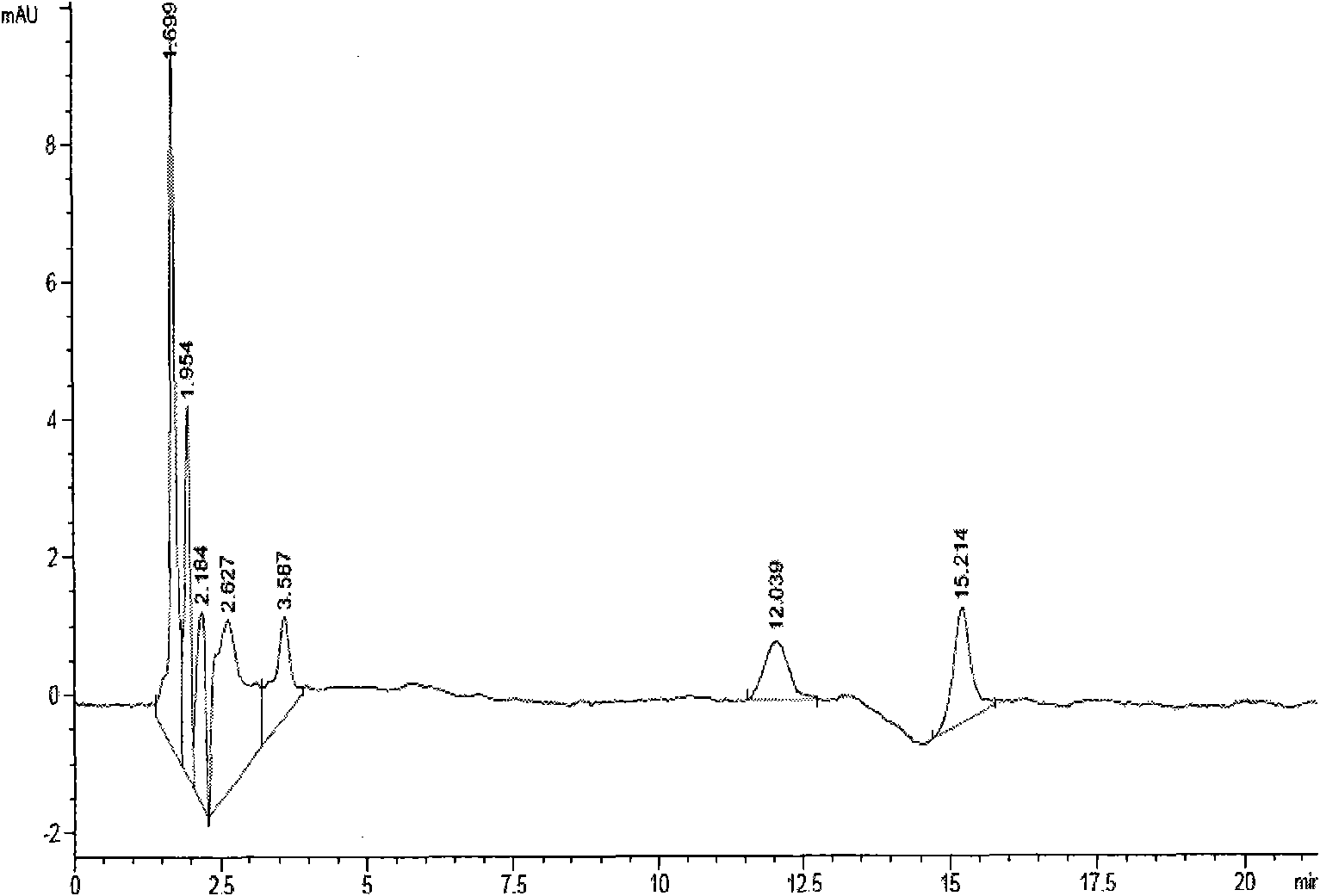
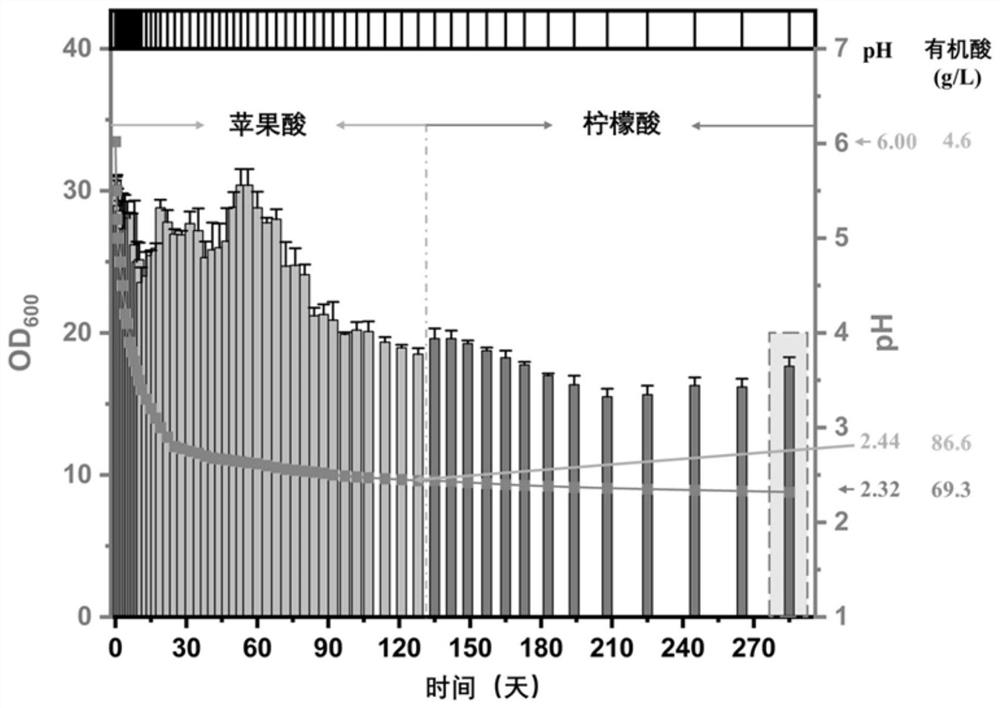
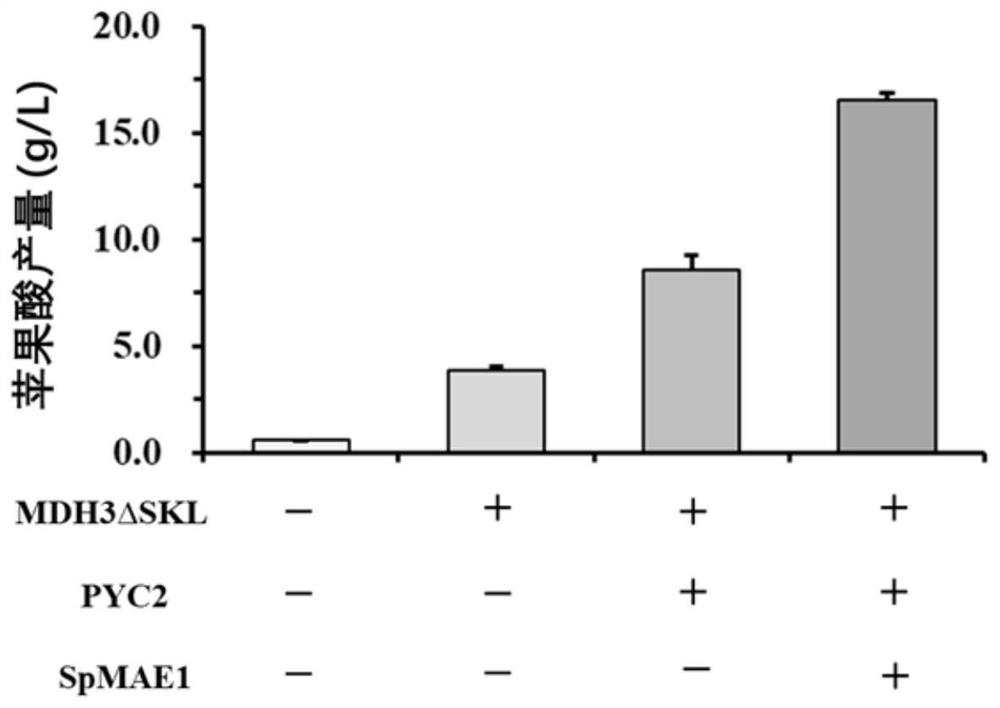
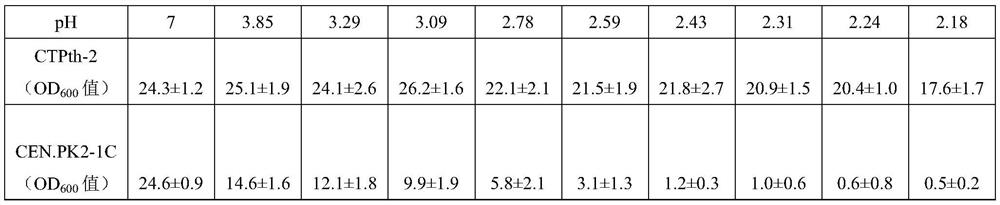
The invention discloses acid-resistant saccharomyces cerevisiae and application thereof in preparation of organic acid. The invention provides a strain of saccharomyces cerevisiae. The saccharomyces cerevisiae is preserved in the China Center for Type Culture Collection, the preservation number is CCTCC M 2021258, and the preservation date is March 19, 2021; the saccharomyces cerevisiae CTPth-2 is obtained through directed evolution screening, and the lowest tolerance pH value is about 2.32, which is also the lowest tolerance pH value of the saccharomyces cerevisiae reported at present; and in order to verify the potential of acid-resistant chassis cell saccharomyces cerevisiae CTPth-2 for producing organic acids, malic acid is taken as an example, a cytoplasm pathway for malic acid synthesis is strengthened, malic acid transporter protein from Schizosaccharomyces pombe is subjected to exogenous expression, an engineering strain CTPM3 is constructed, shake flask fermentation is adopted under the condition that a neutralizer is not added, and the fermentation yield of the malic acid reaches 16.5 g / L.
Owner:JIANGNAN UNIV
Transformant and process for production thereof, and process for production of lactic acid
The present invention relates to a transformant, containing a lactate dehydrogenase gene which is introduced into Schizosaccharomyces pombe as a host, in which a part of a gene cluster encoding a pyruvate decarboxylase in the Schizosaccharomyces pombe host is deleted or inactivated.
Owner:AGC INC
Schizosaccharomyces pombe engineering strain having defensins function and constructing method thereof
InactiveCN101157899AImprove expression levelNormal biological activityFungiOther foreign material introduction processesBiotechnologyProkaryotic expression
The invention provides an engineering bacteria of fission yeast schizosaccharomyces pombe which has a function of defensin and a construction method thereof. A constructed expression carrier of the fission yeast schizosaccharomyces pombe which contains defensin genes is led into the yeast schizosaccharomyces pombe and the fission yeast schizosaccharomyces pombe provided by the invention is obtained. The defensin genes are inserted in the polyclonal locus of the expression carrier of the fission yeast schizosaccharomyces pombe and the constructed expression carrier of the fission yeast schizosaccharomyces pombe is led into the fission yeast schizosaccharomyces pombe to gain recombinant fission yeast schizosaccharomyces pombe which is cultured to get the expression of defensin genes. The invention makes up the shortcomings of the traditional preparation method of defensin and the expression system of pronucleus, saccharomyces cerevisiae and pichia. The invention is applied to the production of industrial defensin and has advantages of large production scale, high activity of defensin, strong sterilizing ability, short growth cycle, and low cost of extraction.
Owner:JILIN AGRICULTURAL UNIV
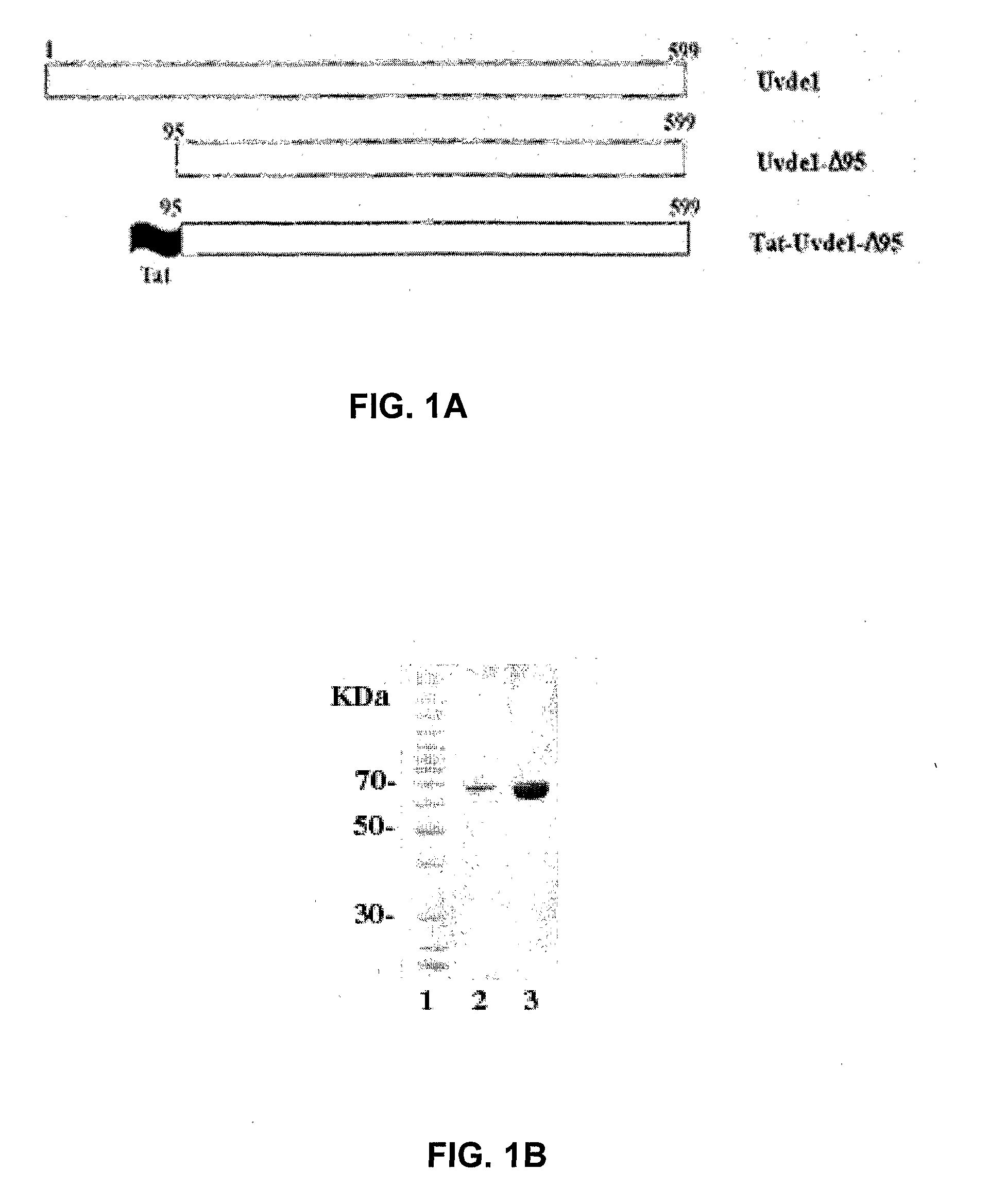
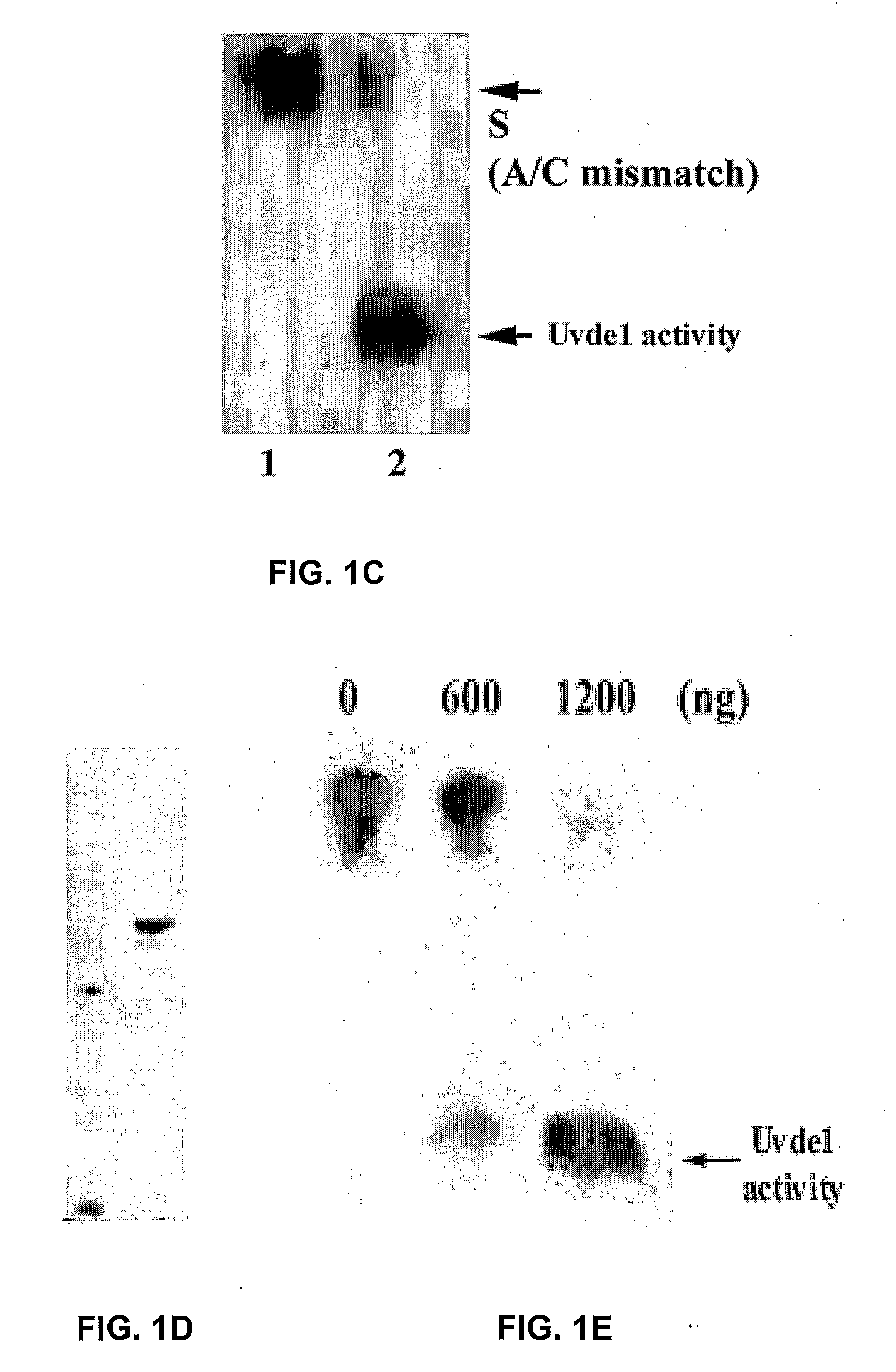
Re-engineered UV damage endonuclease, compositions and methods
InactiveUS20090130034A1Reduce harmStructural damagePolypeptide with localisation/targeting motifCosmetic preparationsUltraviolet lightsCosmeceuticals
Provided are methods and compositions for reducing damage to skin by ultraviolet light and other agents that cause distortion to double stranded DNA and methods for reducing damage to other organs due to such DNA distortion. These compositions comprise a novel truncated UV damage endonuclease (a truncated derivative of Uvde 1 (UVDE, Uvelp) of Schizosaccharomyces pombe) in conjunction with a cell penetrating peptide, together with components suitable for topical application or other administration to a human or animal in need of treatment to reduce damage to due distortion of double-stranded DNA. Methods for reducing DNA distortion-induced damage or deterioration of condition comprise the step of administering a composition comprising the truncated Uvde 1 of the present invention in conjunction with a fused cell penetration peptide or with a noncovalently bound cell penetration peptide to the skin or other organ, or by other route of administration. Compositions for topical application are also useful for cosmetic or cosmeceutical use.
Owner:EMORY UNIVERSITY
Method of Screening Antibacterial Drug Compounds
InactiveUS20100137146A1Increased toxicityBacteriaMicrobiological testing/measurementBacteroidesAntimicrobial drug
Compounds are identified simultaneously as having antibiotic activity targeting a specific microbial protein and having no or limited toxicity against eukaryotic cells by expressing the microbial protein in eukaryotic cells by expressing the microbial protein in eukaryotic cells which then are used to screen candidate antibiotic compounds. Preferably, yeast cells such as Schizosaccharomyces pombe are transfected with and express a target bacterial protein such as FtsZ or MreB, optionally as a fusion with a reporter protein, and these transfected cells are used to screen libraries of compounds simultaneously for activity against the bacterial protein and lack of toxicity against the yeast cell.
Owner:TEMASEK LIFE SCIENCES LABORATORY
Recombinant Vaccine from gE, gI, and gB Proteins of the Varicella-Zoster Virus for the Treatment and Prevention of Multiple Sclerosis
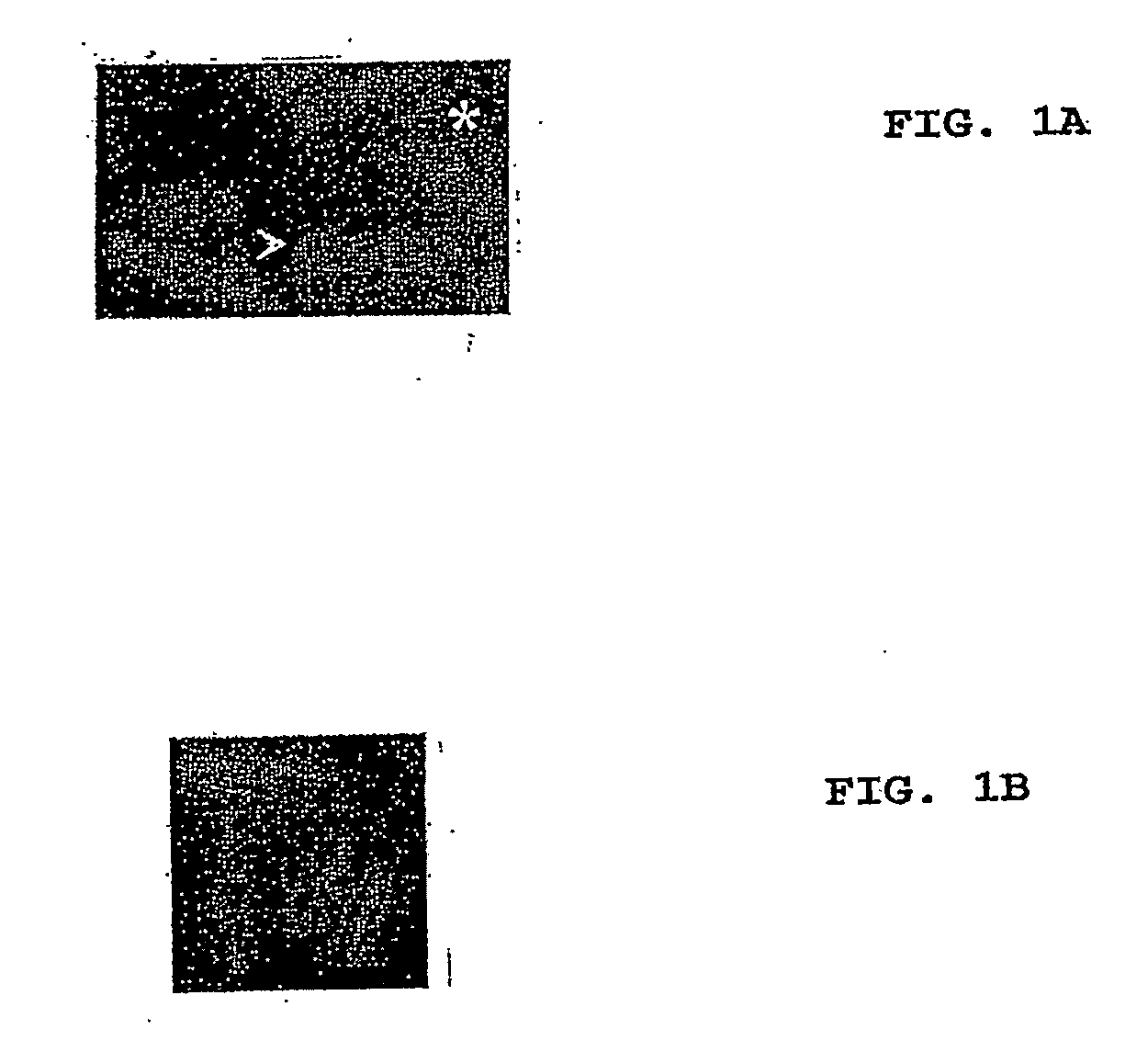
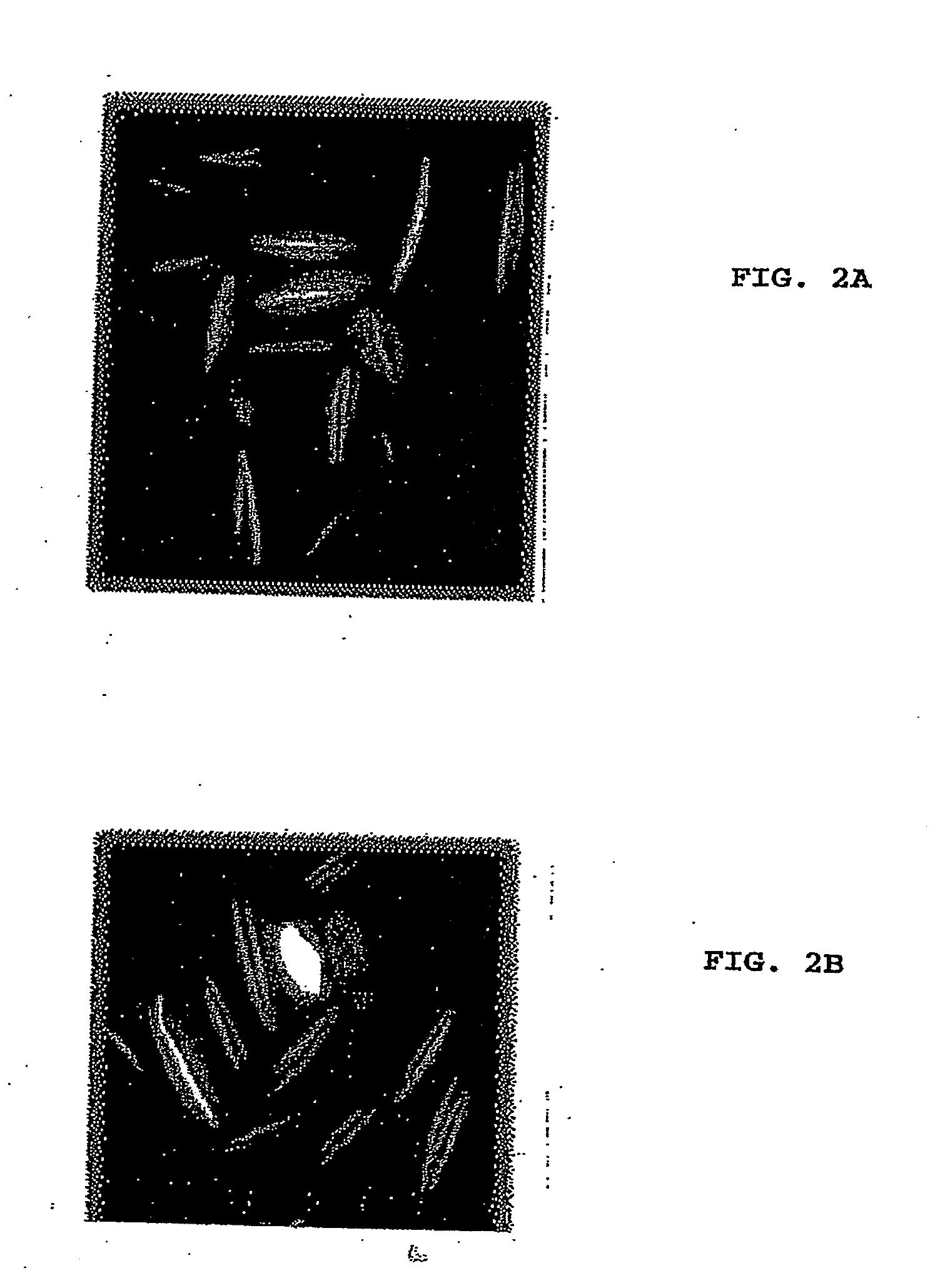
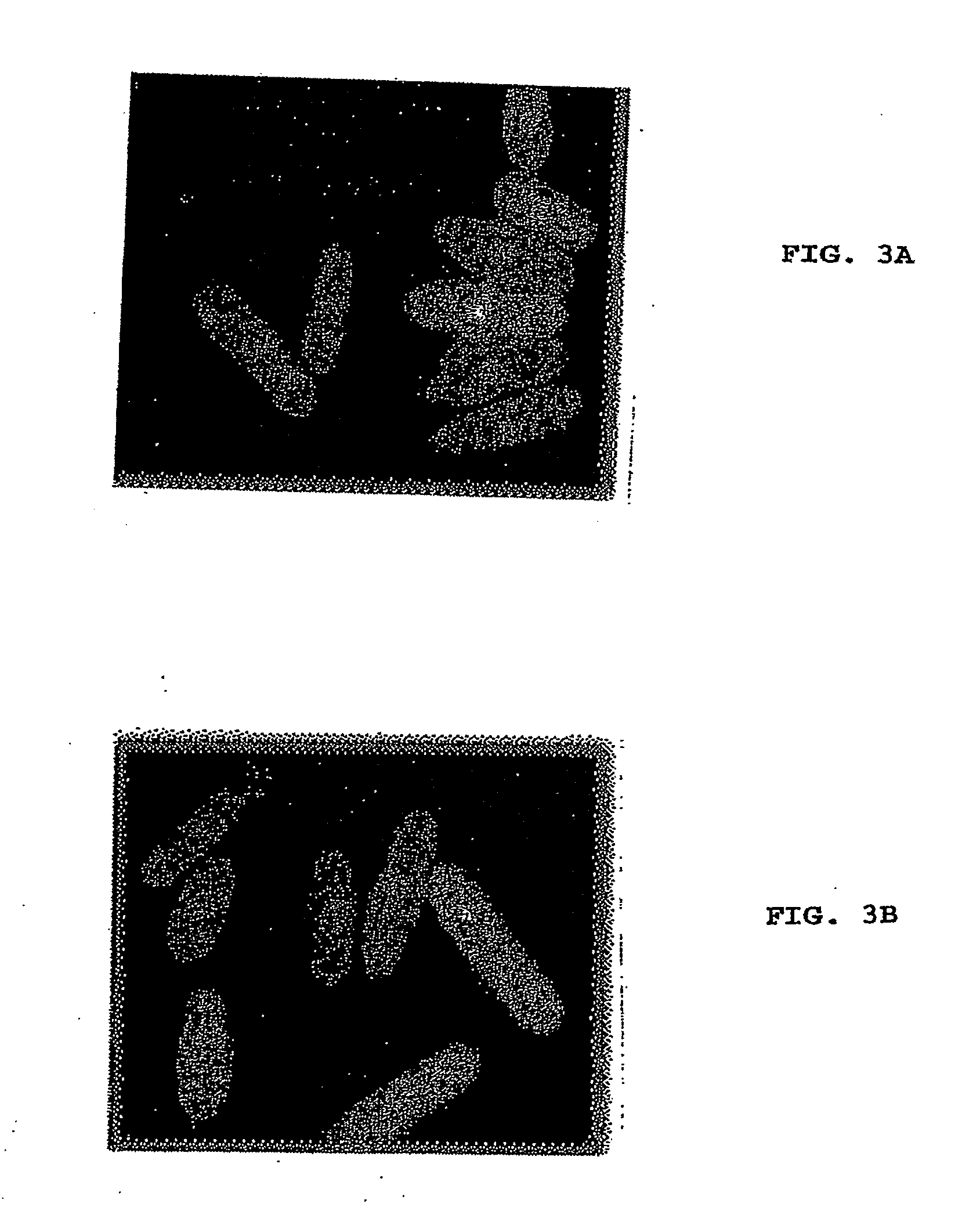
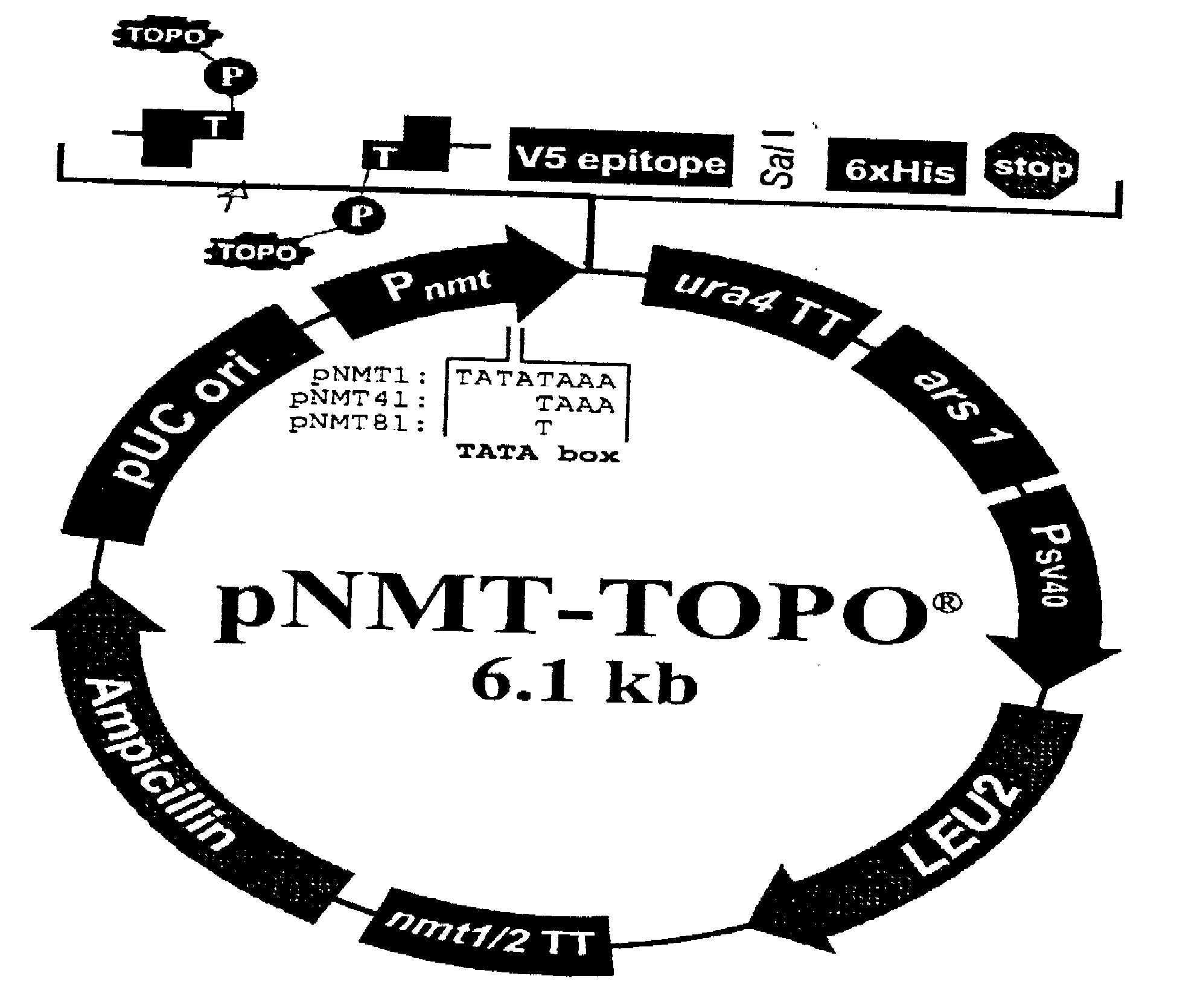
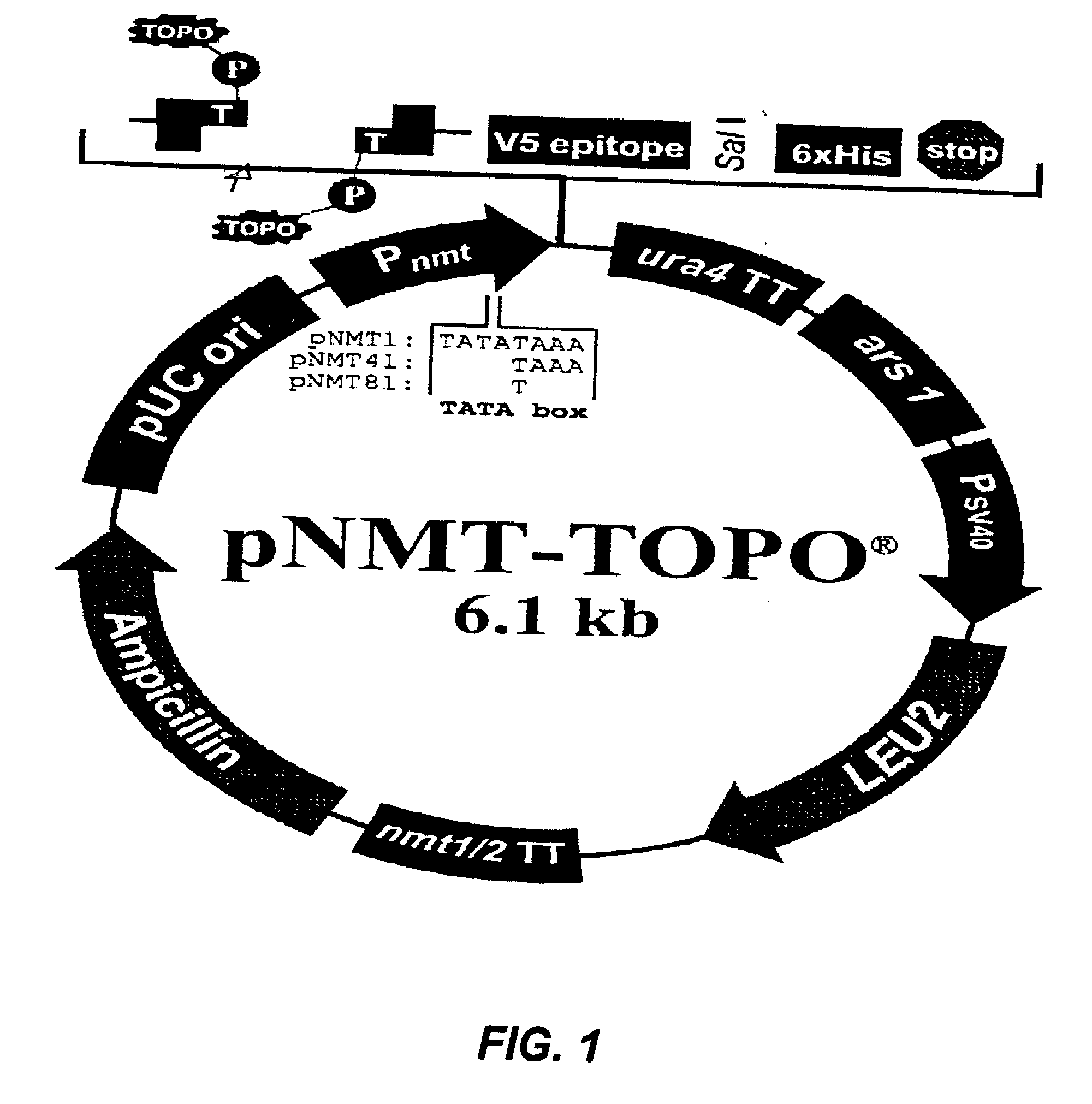
Varicella-zoster virus belongs to the herpesvirus family and its main host are humans, producing 2 different diseases: varicella in children and young adults and zoster in elder or immunodepressed subjects. We reported in the scientific medical literature the unexpected finding that the role of varicella-zoster virus in the pathogeny of Multiple Sclerosis (Archives of Neurology 61: 529-532). This finding allows us to foresee the use of a vaccine against this virus with preventive and therapeutic ends for multiple sclerosis which eventually could also be applicable in the prevention of varicella and zoster. Currently the only vaccine used in humans is that produced by attenuated live varicella-zoster viruses, this latter feature thus avoiding its therapeutic use in multiple sclerosis, wherein the chronic disease is caused by periodic exacerbations of the virus which remains latent in the host, therefore by injecting an attenuated and viable virus the infection may be exacerbated and promote the very latency of the vaccine virus.In our studies the most conspicuous genes of the varicella-zoster virus found in multiple sclerosis patients were the ones corresponding to the genes ORF31 (gB), ORF67 (gI) and ORF68 (gE). The recombinant vaccine which is the subject of this patent is built up by the proteins generated by these genes inserted in a plasmid vector of pNMT1-TOPO in order to transform Schizosaccharomyces pombe and thus obtaining the recombinant viral proteins which build up the vaccine.This vaccine, by being made from recombinant viral proteins eliminates the risks associated to the use of vaccines from attenuated viable viruses. Likewise, the use of these recombinant viral proteins is specific and sensitive to serological tests for the diagnosis of infections caused by the varicella-zoster virus.
Owner:SOTELO MORALES JULIO EVERARDO +2
Probe for absolute quantification of schizosaccharomyces pombe, kit and application
ActiveCN112779349ARealize the total detectionQuick checkMicrobiological testing/measurementMicroorganism based processesBiotechnologySchizosaccharomyce pombe
The invention discloses a probe for absolute quantification of schizosaccharomyces pombe, a kit and application, and belongs to the fields of biology, fermentation and detection. According to the probe for absolute quantification of schizosaccharomyces pombe and the kit, the total amount of schizosaccharomyces pombe can be detected, and can be used for detecting and quantifying the schizosaccharomyces pombe without expensive instruments, and the quantification work can be rapidly completed within 2.5h. Meanwhile, the sample used in the present invention need no nucleic acid extraction. Quantification of schizosaccharomyces pombe based on the probe and the detection kit provided by the invention has the characteristics of rapidness, convenience, cheapness and accuracy.
Owner:JIANGNAN UNIV
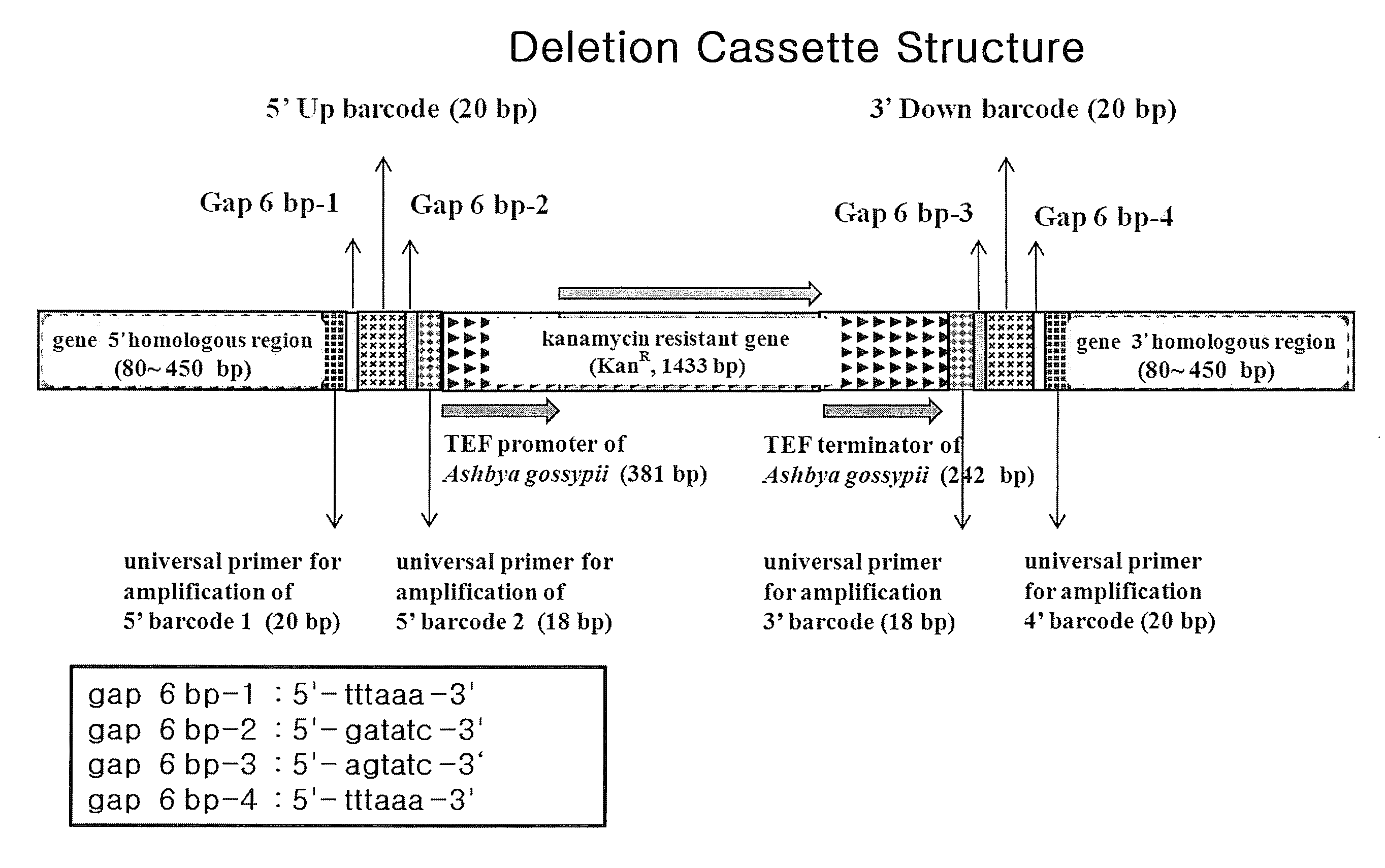
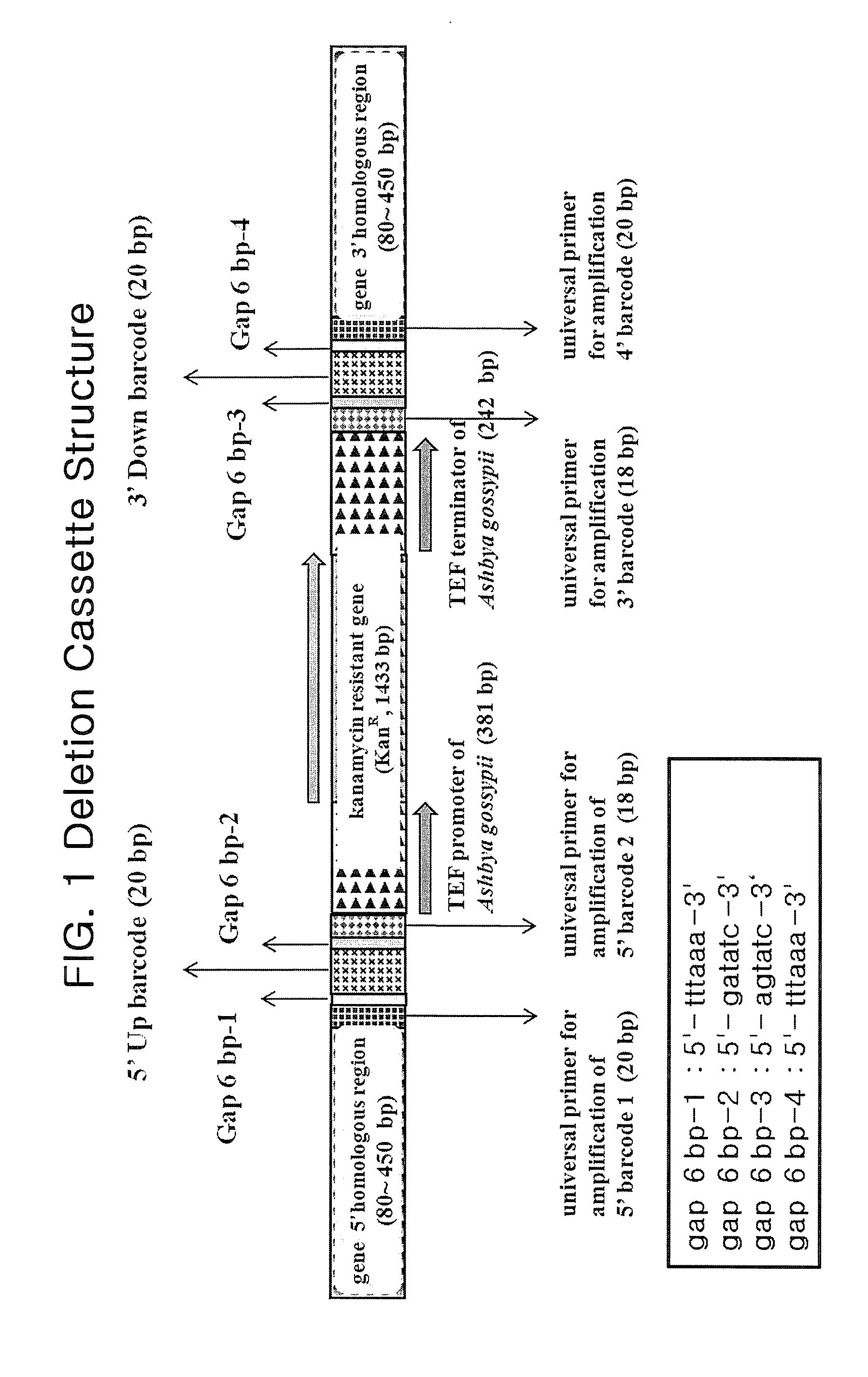
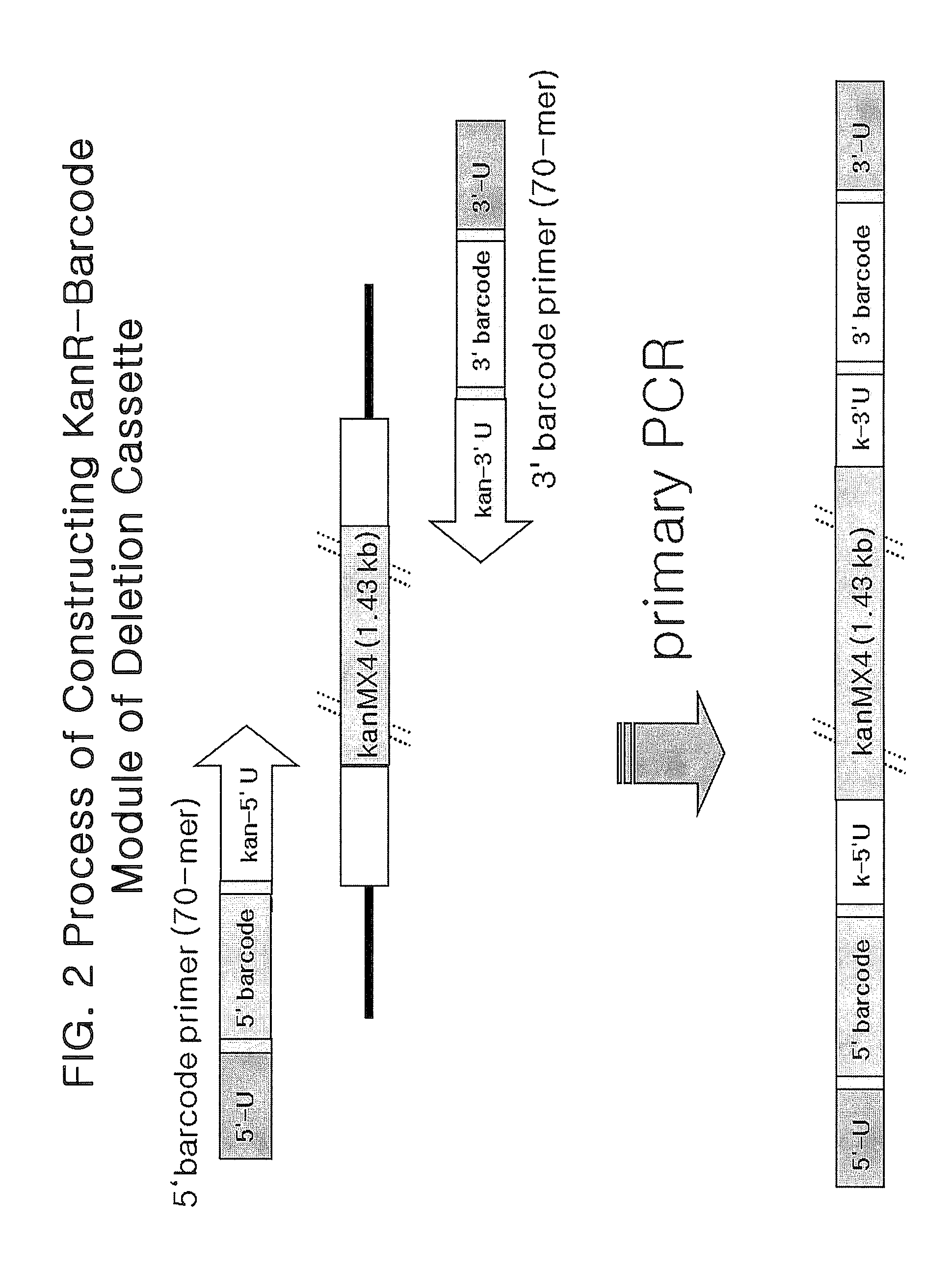
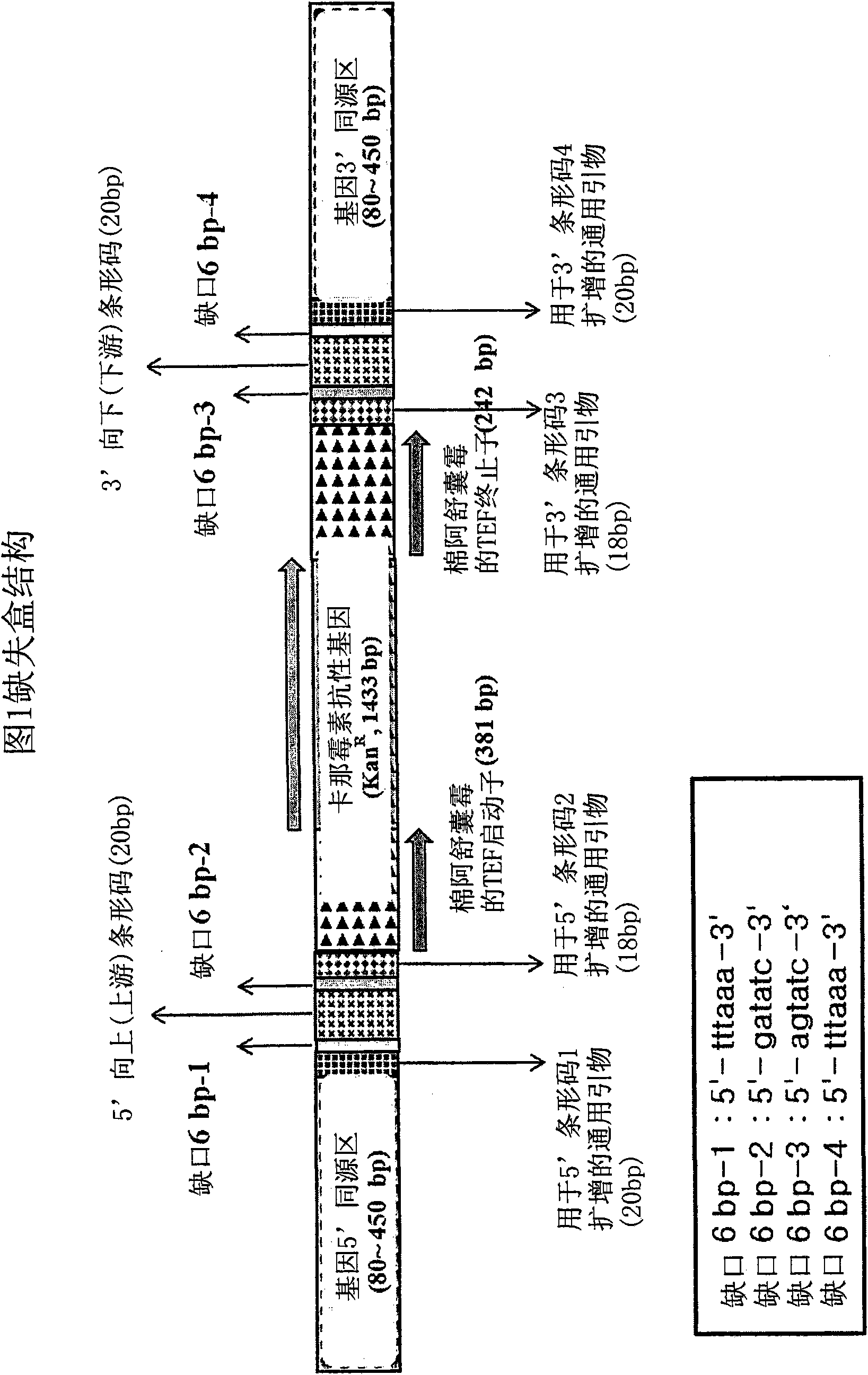
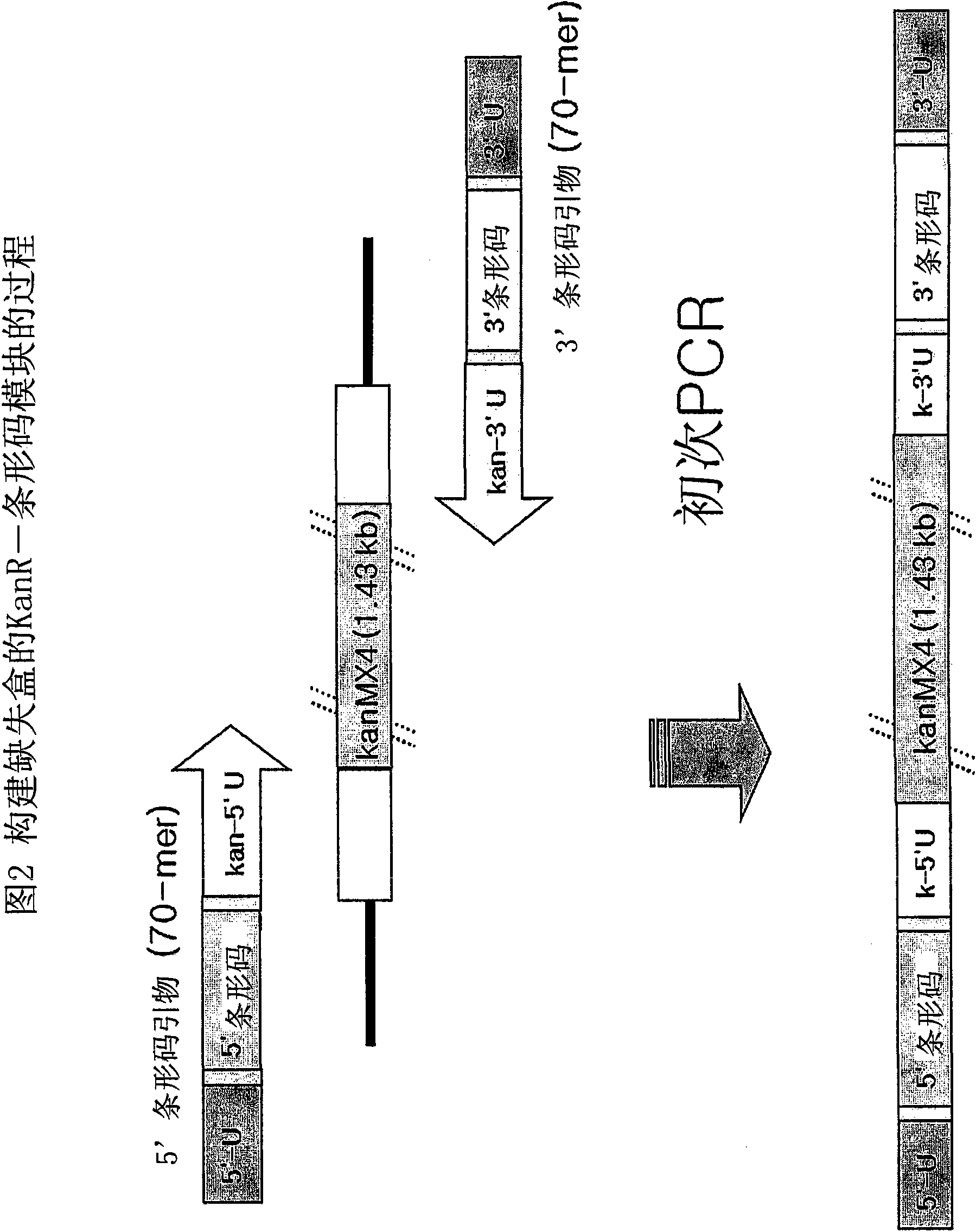
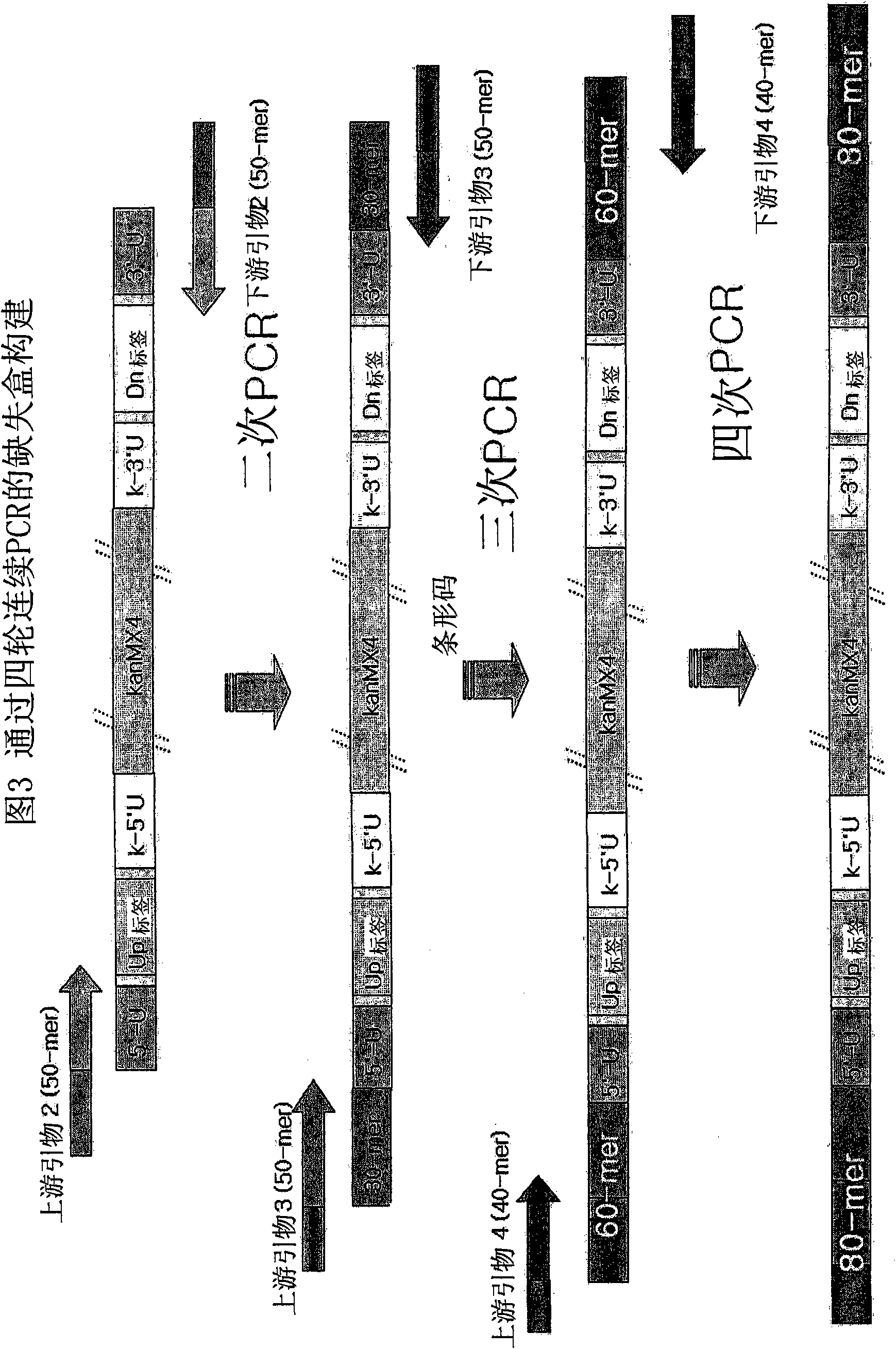
Genome-Wide Construction of Schizosaccharomyces Pombe Heterozygous Deletion Mutants Containing Gene-Specific Barcodes by the Methods of 4-Round Serial or Block PCR, or Total Gene Synthesis Thereof
ActiveUS20110190163A1Increase success rateEfficient developmentFungiLibrary screeningMode of actionSchizosaccharomyces pombe
A method comprising transforming Schizosaccharomyces pombe with a deletion cassette, constructed by four-round serial PCR, block PCR or total gene synthesis, containing a homologous recombination site is provided for preparing gene-targeted heterozygous deletion Schizosaccharomyces pombe. Also provided are gene-targeted hetero2ygous deletion Schizosaccharomyces pombe mutants prepared by the method, and a library of gene-targeted heterozygous deletion Schizosaccharomyces pombe mutants. Further, the library is useful in constructing a method and a kit for screening a drug's modes of action.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Histone Demethylation Mediated by the Nuclear Amine Oxidase Homolog LSD1
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Genome-wide construction of schizosaccharomyces pombe heterozygous deletion mutants containing gene-specific barcodes by the methods of 4-round serial or block pcr, or total gene synthesis thereof
A method comprising transforming Schizosaccharomyces pombe with a deletion cassette, constructed by four-round serial PCR, block PCR or total gene synthesis, containing a homologous recombination site is provided for preparing gene-targeted heterozygous deletion Schizosaccharomyces pombe. Also provided are gene-targeted hetero2ygous deletion Schizosaccharomyces pombe mutants prepared by the method, and a library of gene-targeted heterozygous deletion Schizosaccharomyces pombe mutants. Further, the library is useful in constructing a method and a kit for screening a drug's modes of action.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Kiwi fruit wine and preparation process thereof
ActiveCN107057928AExcellent preparation efficiencyGood quality fruit wineAlcoholic beverage preparationFruit wineFlavor
The invention relates to the technical field of wine making, in particular to a kiwi fruit wine and a preparation process thereof. The preparation process comprises the following step: fermenting kiwi fruits by using schizosaccharomyces promb. The kiwi fruit wine is prepared by the preparation process. The screened schizosaccharomyces promb is used as a fermenting strain, the quality of the fruit wine is ensured, and the optimal process parameters for production of Hongyang kiwi fruit wine are determined. The finally prepared kiwi fruit wine is moderate in alcoholic strength, full in kiwi fruit flavor and obvious in wine fragrance, and is fresh, cool and delicious; and the provided preparation process is high in production efficiency, and meets requirements of industrialized production of the kiwi fruit wine.
Owner:SICHUAN AGRI UNIV
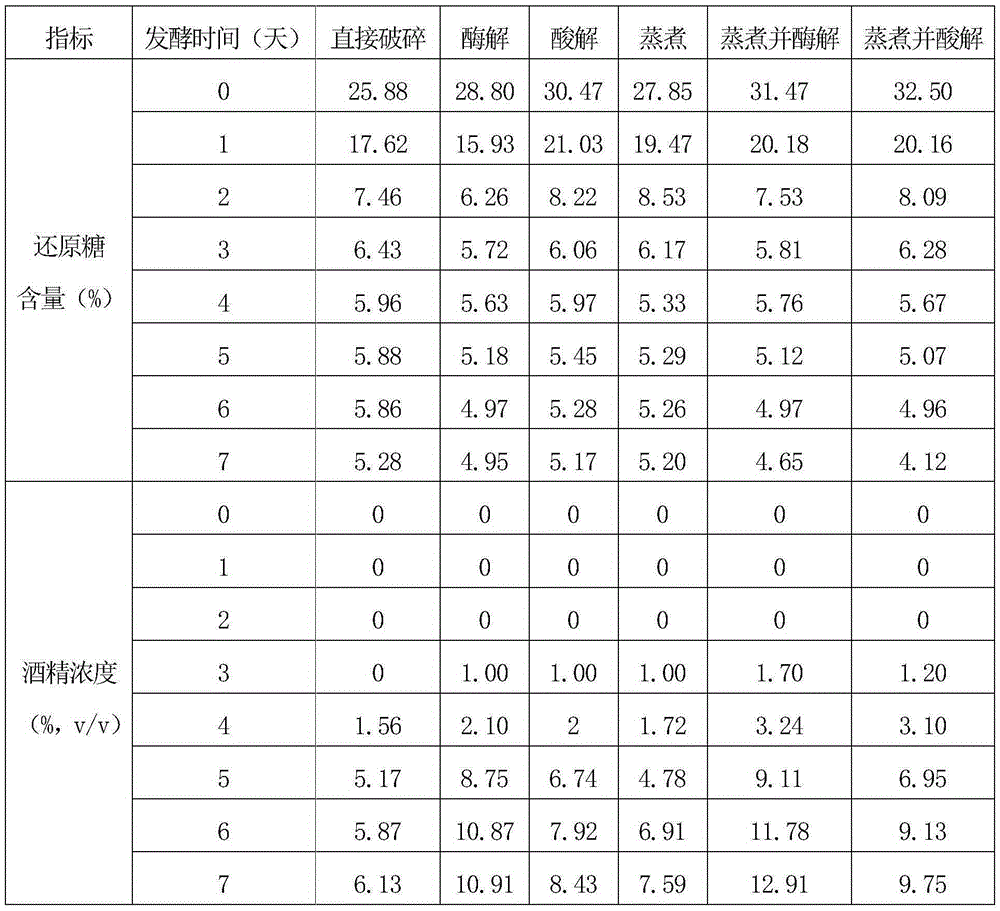
Edible alcohol fermentation production process
InactiveCN107245411AReduced nutrient contentGreat tasteAlcoholic beverage preparationMicroorganism based processesPectinaseNutritive values
The invention discloses an edible alcohol fermentation production process. The edible alcohol fermentation production process comprises the following steps that longans and red dates are prepared according to the weight part ratio of 1:10 to 1:3; the raw materials longans and red dates are cooked in a steam cooking pot according to the ratio, then tap water is added into the longans and red dates which are cooked, even stirring is performed, and then smaller lumped koji powder is added and evenly stirred to make thick mash; the thick mash is added into a saccharifying enzymolysis tank, and then smaller lumped koji is added and evenly stirred for saccharification; then the mixture is transferred into an enzymolysis tank, aspergillus niger pectinase is added, and enzymolysis is performed to obtain secondary thick mash; a schellozosaccharomyces pombe strain is inoculated to the secondary thick mash, then the secondary thick mash is put in an alcohol fermentation tank, peptone is added, and stirring and fermentation are performed to obtain fermented mash; the fermented mash is distilled by using a five-tower secondary differential-pressure distillation device to obtain an edible alcohol finished product. The brewed edible alcohol has good qi tonifying and blood benefiting effects, has a higher nutritive value and has a sweet and good taste.
Owner:崇州市富易生物科技有限公司
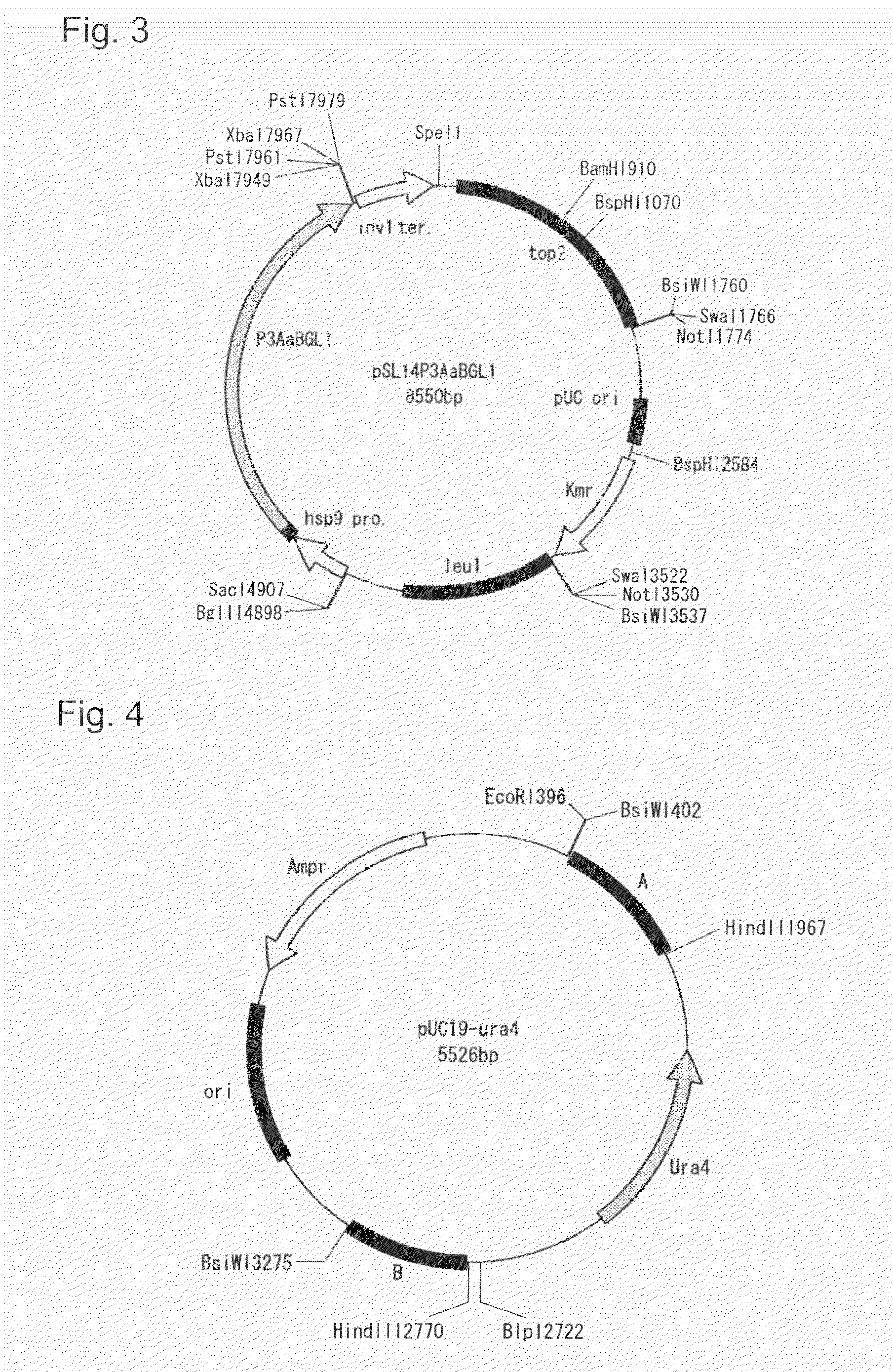
Transformant of schizosaccharomyces pombe mutant and cloning vector
Owner:ASAHI GLASS CO LTD
Method for transforming schizosaccharomyces pombe, transformant of schizosaccharomyces pombe and method for producing heterologous protein
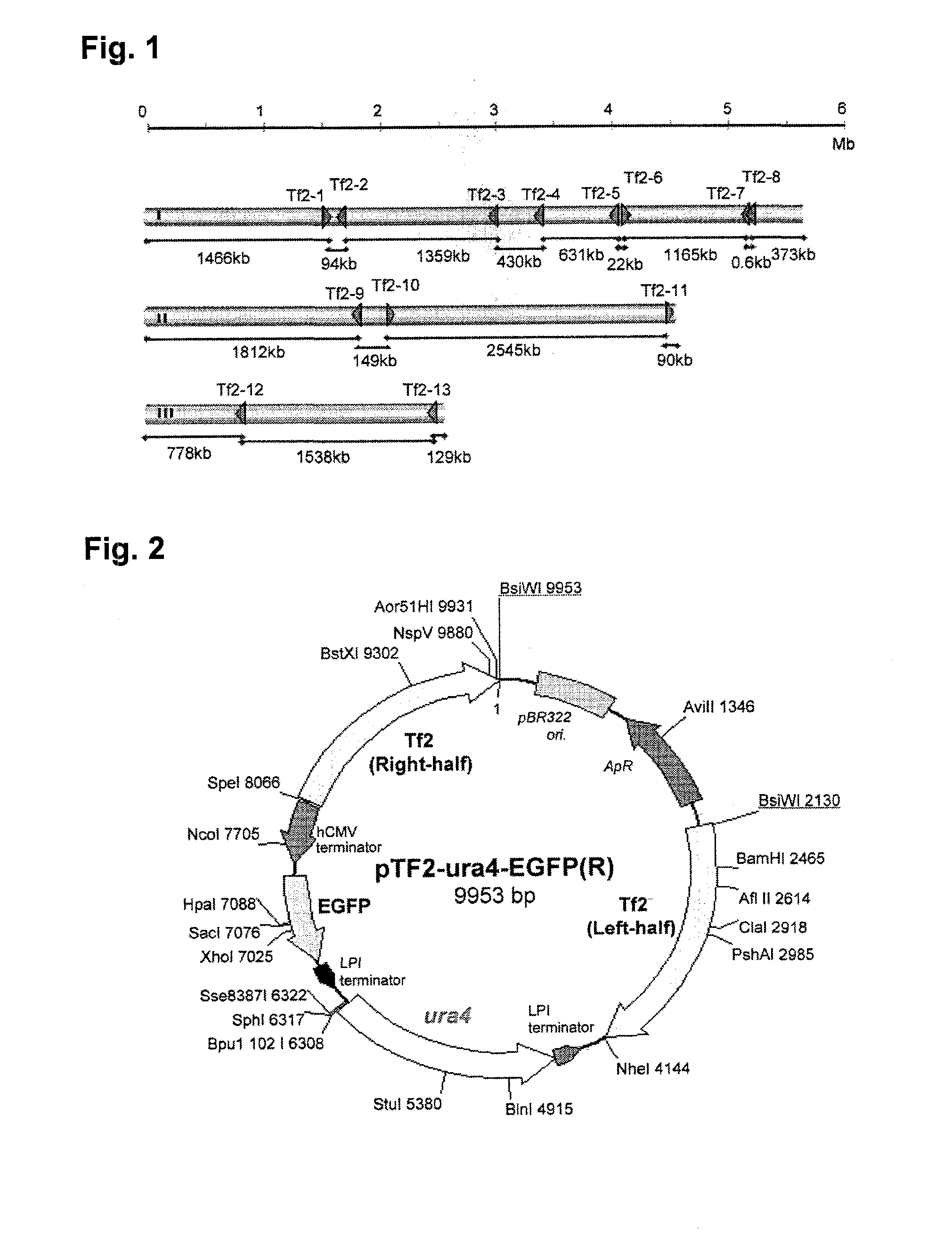
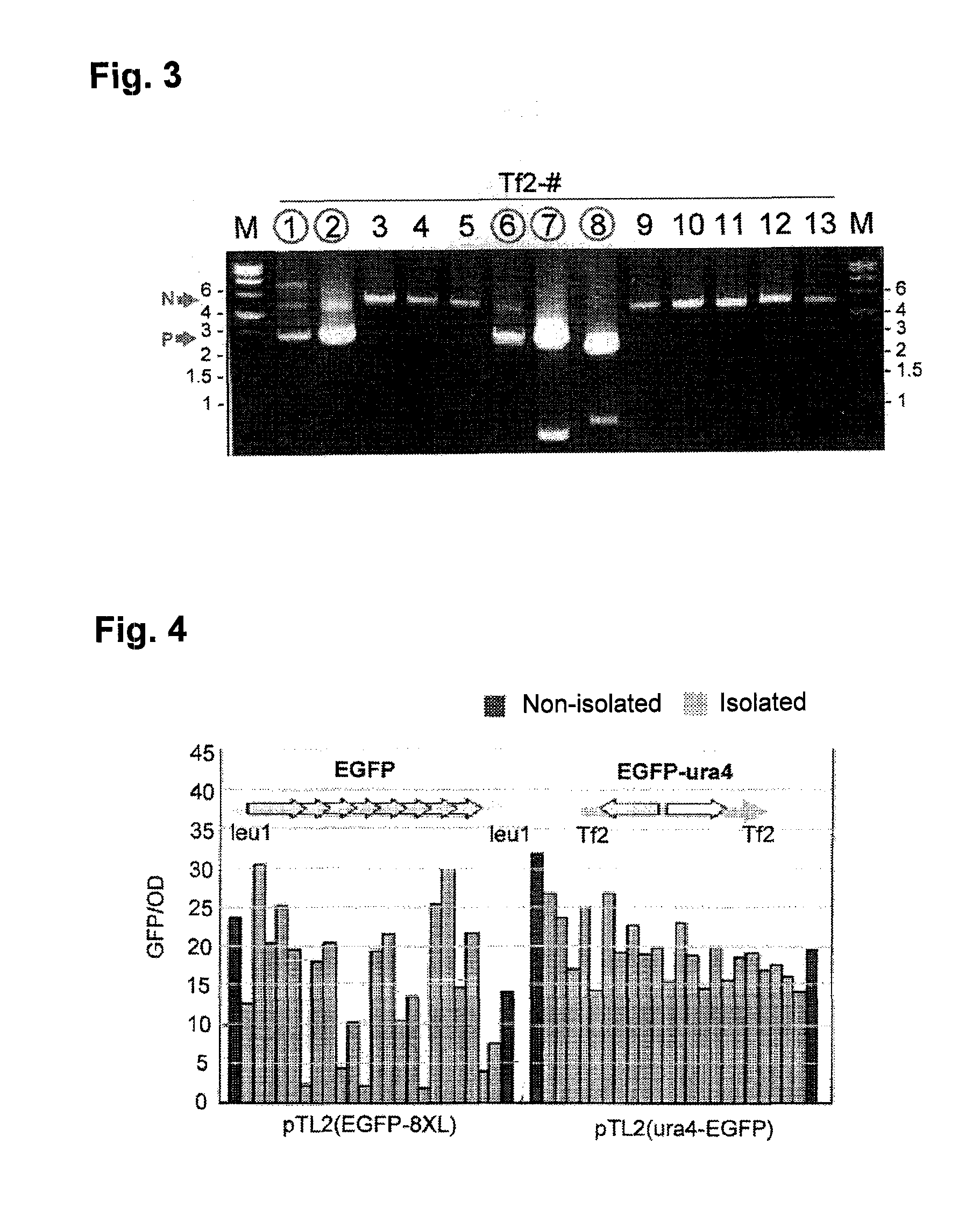
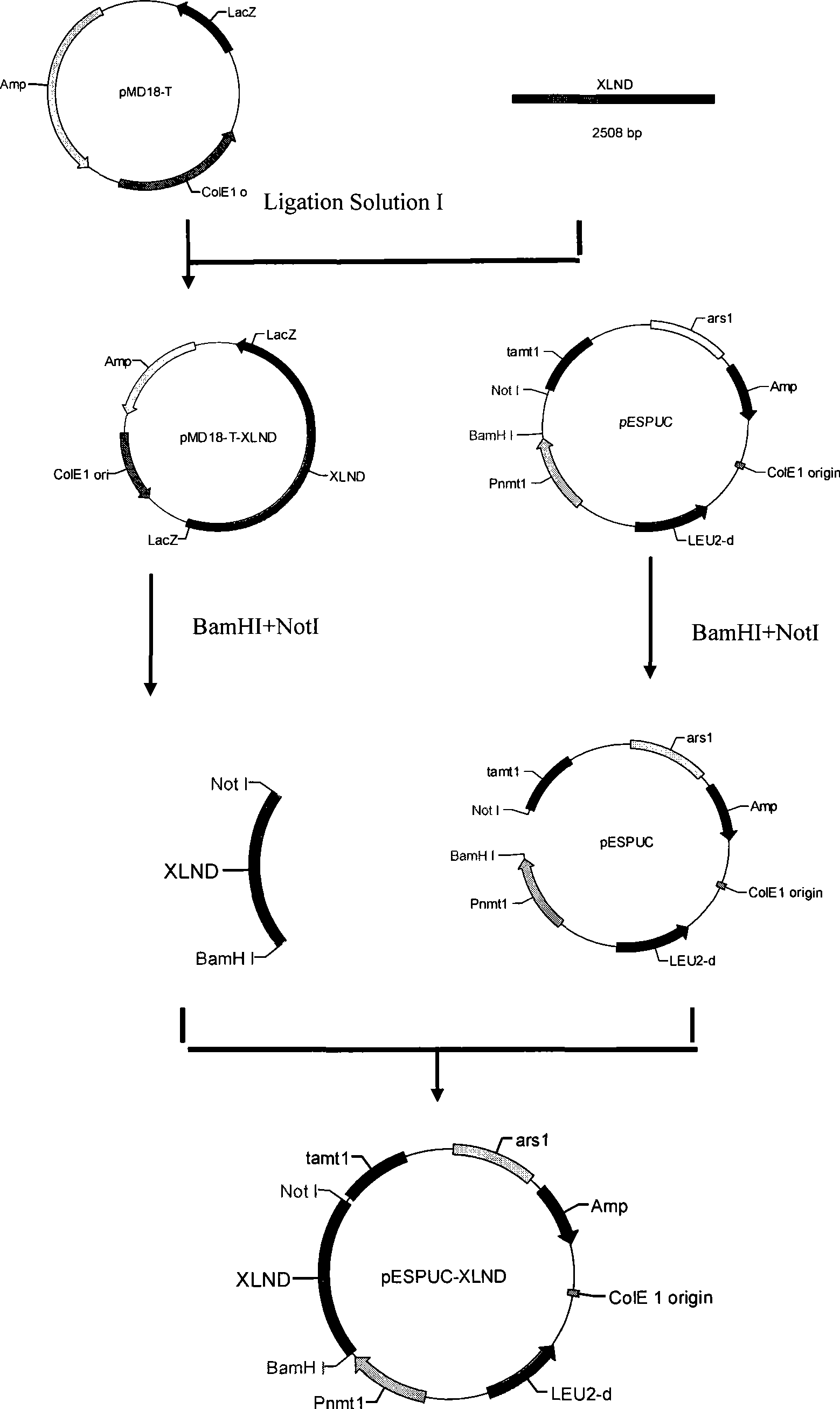
To provide a method for transforming S. pombe for creating a transformant with a high stability of maintenance after passage and enables the steady production of a heterologous protein of interest, a transformant produced by the method and a method for producing a heterologous protein using the resultant transformant.A method for transforming Schizosaccharomyces pombe by using a vector carrying an expression cassette (containing a promoter capable of functioning in Schizosaccharomyces pombe, a heterologous protein structural gene and a terminator), and having recombination region(s) at which homologous recombination with each chromosome of Schizosaccharomyces pombe is to be achieved, which comprises integrating the expression cassette into the Tf2 transposon gene position(s) in the chromosome(s) of Schizosaccharomyces pombe by homologous recombination, a transformant created by the method and a method for producing a heterologous protein using the resultant transformant.
Owner:ASAHI GLASS CO LTD
Schizosaccharomyces pombe engineering bacteria having hemicellulase activity and construction method thereof
The invention relates to a Schizosaccharomyces pombe engineering bacteria with hemicellulase activity and a method for constructing the same. The Schizosaccharomyces pombe engineering bacteria is obtained by introducing a constructed recombinant Schizosaccharomyces pombe expression vector containing hemicellulase gene into Schizosaccharomyces pombe; and the construction method comprises the following steps: constructing a Schizosaccharomyces pombe expression vector containing the hemicellulase gene; introducing the Schizosaccharomyces pombe expression vector into the Schizosaccharomyces pombe to obtain a recombinant yeast transformant; and cultivating a recombinant yeast to secrete and express the enzyme gene of the hemicellulase. The invention makes up the defects of the prior method for preparing the hemicellulase, a prokaryotic expression system, and other yeast expression systems. When the Schizosaccharomyces pombe engineering bacteria is applied to the industrial production of the hemicellulase, the Schizosaccharomyces pombe engineering bacteria has the advantages of high enzyme activity, strong thermal stability, short growth cycle, and low extraction cost.
Owner:JILIN AGRICULTURAL UNIV
Screening method for drug target gene using heterozygous deletion fission yeast strain
InactiveUS20150344874A1Easy to useGood choiceMicrobiological testing/measurementLibrary screeningBudding yeastsScreening method
The present invention relates to a screening method for a drug target gene by using chemical-genetic profile compendium of the heterozygous deletion fission yeast strain and the comparative genetic analysis using the same. More precisely, the present inventors constructed the chemical-genetic profile compendium for drug candidates from the heterozygous deletion fission yeast strain of Schizosaccharomyces pombe (S. pombe), and then compared with the compendium with the chemical-genetic profile compendium of the budding yeast Saccharomyces cerevisiae (S. cerevisiae) in order to select efficiently drug target genes showing drug sensitivity. The screening method of the present invention can be efficiently used for the identification of a drug target gene in various eukaryotes because it facilitates the selection of a drug target gene showing sensitivity to the drug from the chemical-genetic profile compendium of the heterozygous deletion fission yeast strain.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Transformant and process for production thereof, and process for production of lactic acid
ActiveUS20190153453A1Improve productivitySuitable for productionOxidoreductasesFermentationBacteroidesYeast
The present invention relates to a transformant which uses Schizosaccharomyces pombe as a host into which a D-LDH gene derived from bacteria of the genus Pediococcus and a D-LDH gene derived from bacteria of the genus Lactobacillus are incorporated and in which some of the genes in a group of pyruvate decarboxylase-encoding genes of the Schizosaccharomyces pombe host have been deleted or inactivated.
Owner:JAPAN MATERIAL TECH CORP
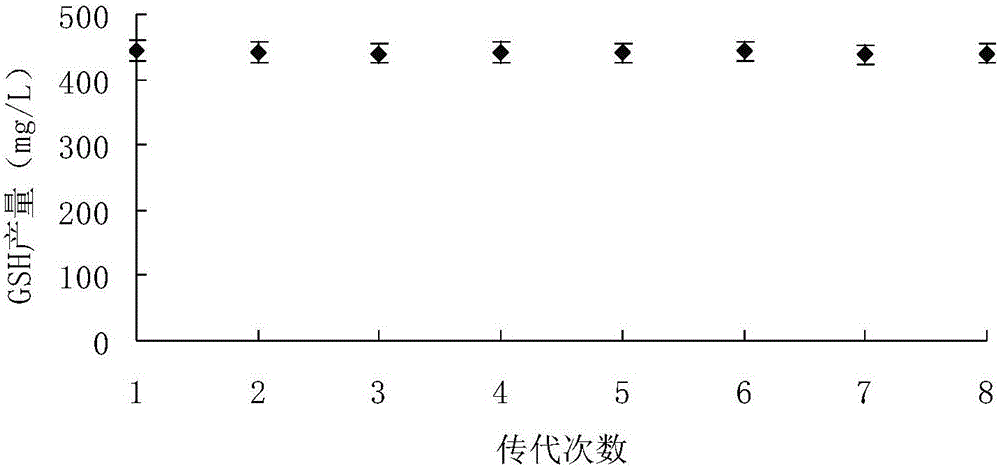
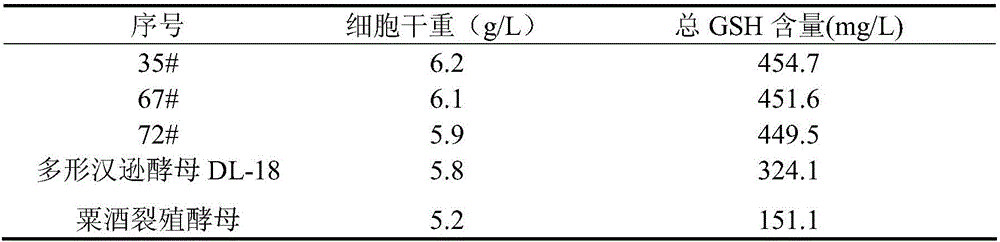
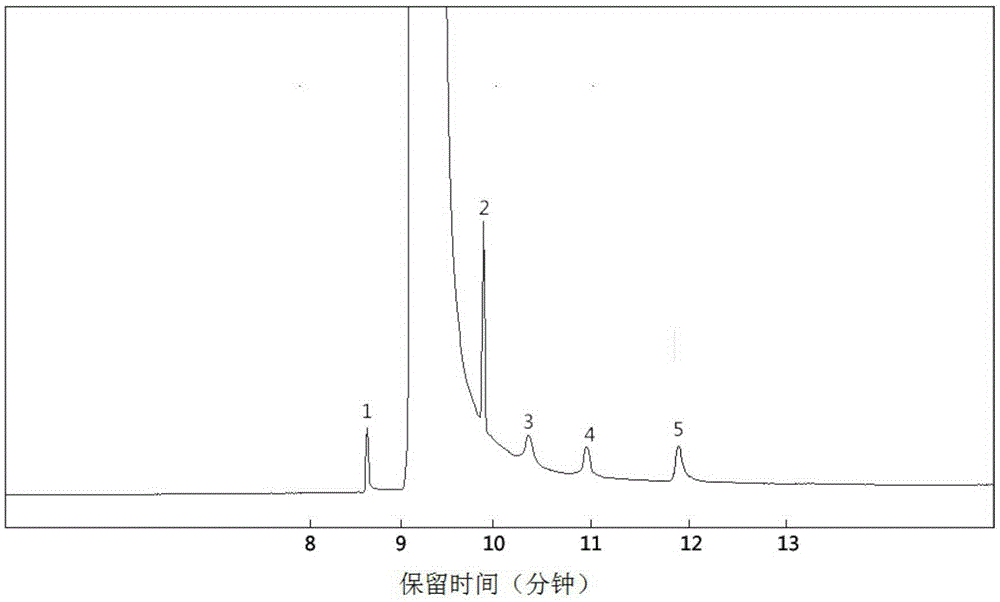
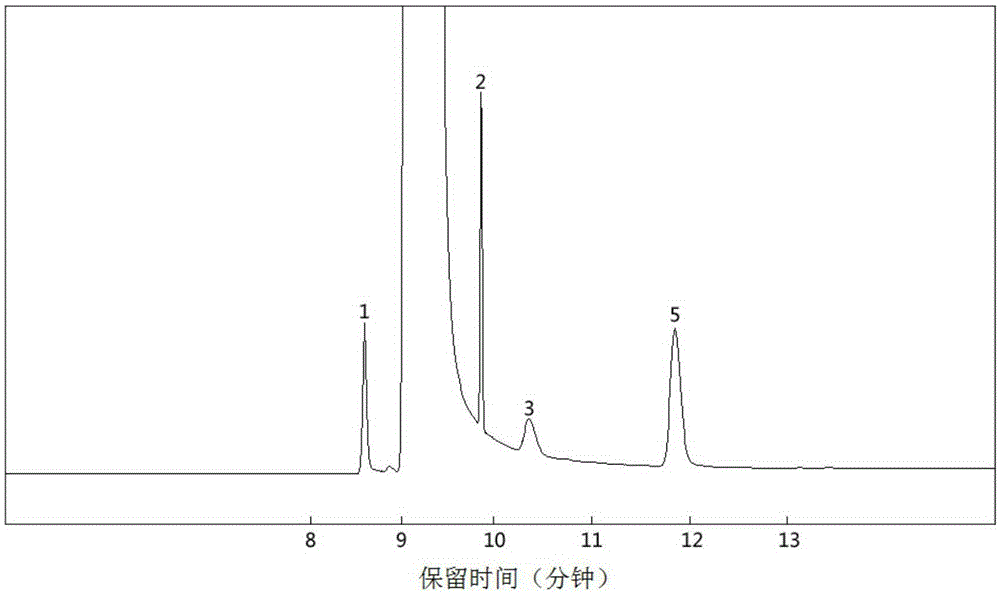
Method for preparing yeast fusants and screening glutathione high-yield strains
ActiveCN105838706AHigh temperature resistance hasFast growthMutant preparationMicroorganism based processesSodium molybdateScreening method
The invention provides a method for preparing yeast fusants and screening glutathione high-yield strains. The method comprises the following steps: respectively preparing protoplasts of hansenula polymorpha and schizosaccharomyces pombe; fusing the protoplasts to obtain fusion processing cells; performing high temperature resistance, ethanol tolerance and sodium molybdate tolerance screening on the fusion processing cells to obtain the glutathione high-yield strains. According to the method, the fusants of the high-yield glutathione can be screened efficiently; the fusants obtained by screening have the advantages of high temperature resistance, high growth rate, genetic stability, high yield of the glutathione, and the like; the strains with application value are provided for the large-scale production of the glutathione.
Owner:SHAANXI UNIV OF SCI & TECH
Method for producing edible alcohol by high-temperature fermentation of defective jujube thick mash
ActiveCN104046542BComply with hygienic standardsAlcoholic beverage preparationMicroorganism based processesAlcoholSchizosaccharomyce pombe
The invention discloses a method for producing edible alcohol by fermenting thick mash of defective red dates at a high temperature. According to the advantages of high sugar content of defective red dates, defective red dates are taken as raw materials, by virtue of high-temperature alcohol-resistant Schizosaccharomyces pombe, and the thick mash of red dates is subjected to high-temperature alcoholic fermentation to obtain red dates edible alcohol dates. According to the method, the conversion rate from carbohydrates in red dates to ethanol reaches above 80%, and the produced red dates edible alcohol is in line with hygienic standards of national edible alcohol.
Owner:SHAANXI NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com