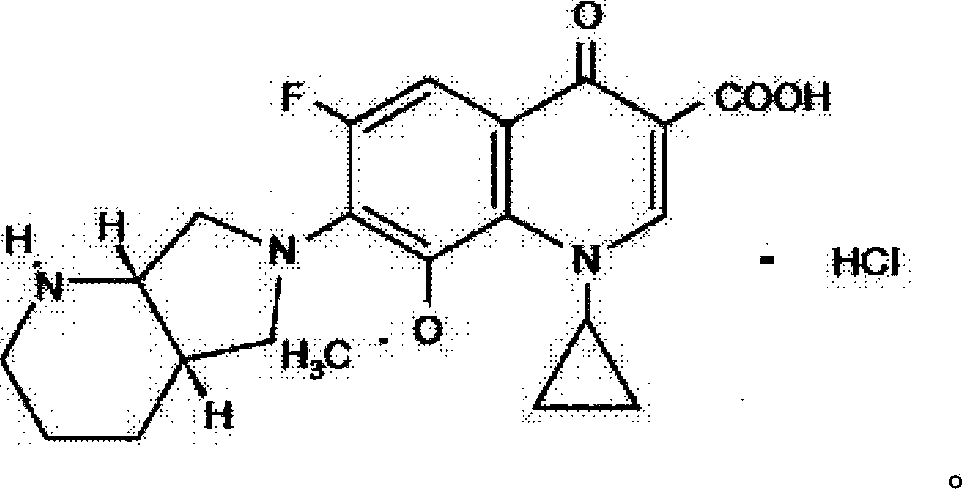
Moxifloxacin hydrochloride pharmaceutical composition and preparation method thereof
A technology of moxifloxacin hydrochloride and its composition, which is applied in the field of medicine, can solve problems such as difficult control, complex process, and uneven coating film, and achieve the goals of saving usage and cost, good quality stability, and shortening coating time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
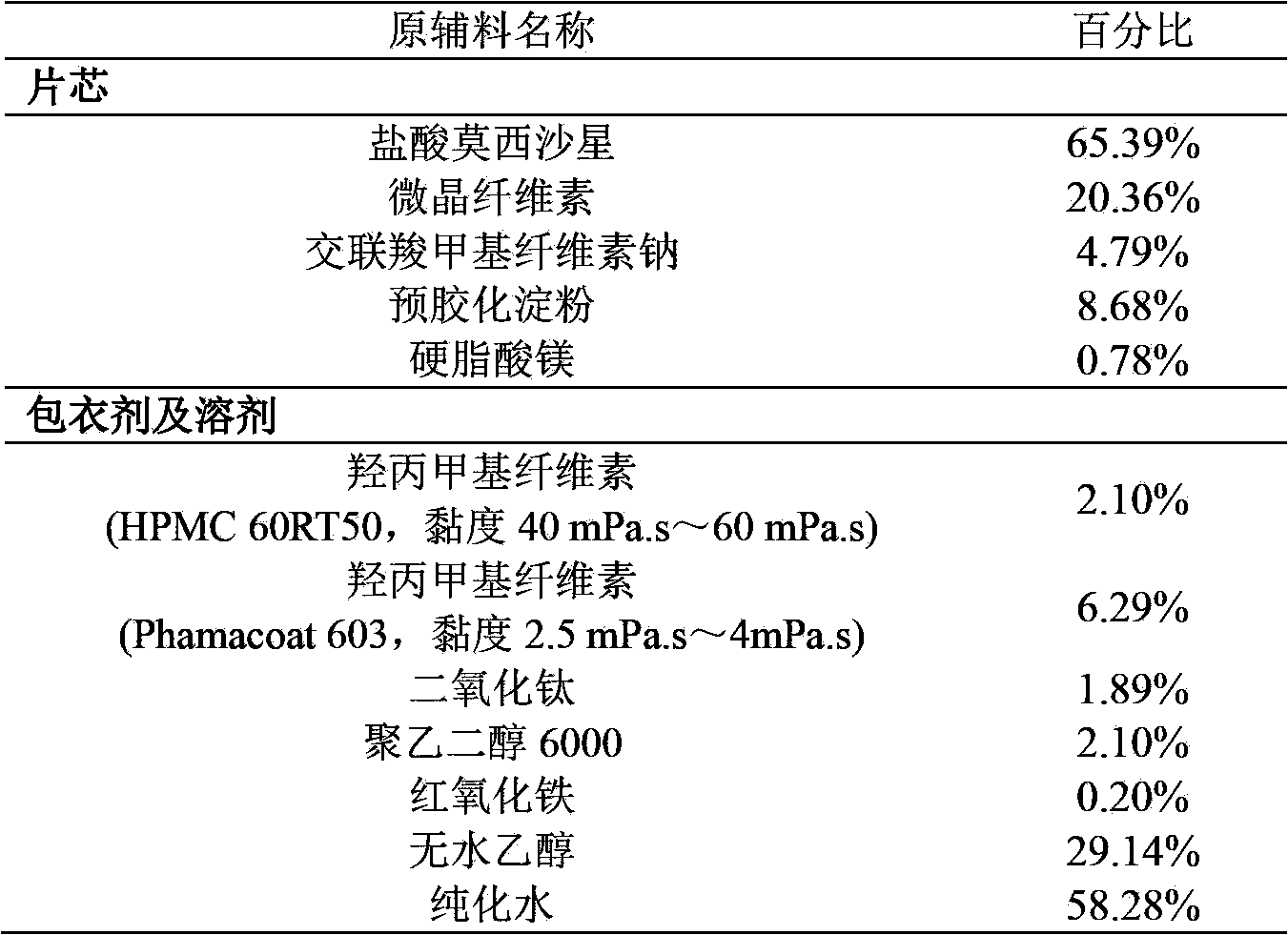
[0052] Embodiment 1 moxifloxacin hydrochloride pharmaceutical composition
[0053]
[0054] Tablet core preparation method: mix moxifloxacin hydrochloride, microcrystalline cellulose, croscarmellose sodium, and pregelatinized starch evenly, add moxifloxacin hydrochloride, microcrystalline cellulose, and croscarmellose Sodium and water with 45% of the total weight of pregelatinized starch are wet granulated. After drying and granulating, the dry granules are mixed with magnesium stearate evenly, and the tablet core is obtained by pressing according to the content of the granules. Each tablet is equivalent to molybdenum. Cifloxacin 400mg;
[0055] Coating: Mix two kinds of hypromellose, polyethylene glycol 6000, titanium dioxide and red iron oxide evenly, add them to absolute ethanol to disperse evenly, and then add purified water under stirring conditions to obtain a coating solution , place the tablet core in a coating pan, control the temperature of the material at 40°C f...
Embodiment 2
[0056] Embodiment 2 moxifloxacin hydrochloride pharmaceutical composition
[0057]
[0058] Tablet core preparation method: mix moxifloxacin hydrochloride, microcrystalline cellulose, croscarmellose sodium, and pregelatinized starch evenly, add moxifloxacin hydrochloride, microcrystalline cellulose, and croscarmellose Sodium and water with 50% of the total weight of pregelatinized starch are wet granulated. After drying and granulating, the dry granules are mixed with magnesium stearate evenly, and the tablet core is obtained by pressing according to the content of the granules. Each tablet is equivalent to molybdenum. Cifloxacin 400mg;
[0059] Coating: Mix two kinds of hypromellose, macrogol 6000, titanium dioxide and red iron oxide evenly, add them to the mixed solvent of absolute ethanol and purified water to disperse evenly, stir to prepare the coating solution, and Tablet cores were placed in a coating pan, the temperature of the material was controlled at 50°C for c...
Embodiment 3
[0060] Embodiment 3 moxifloxacin hydrochloride pharmaceutical composition
[0061]
[0062]
[0063] Tablet core preparation method: mix moxifloxacin hydrochloride, calcium phosphate, and cross-linked polyvinylpyrrolidone evenly, add water with 40% of the total weight of moxifloxacin hydrochloride, calcium phosphate, and cross-linked polyvinylpyrrolidone, perform wet granulation, and dry the granules Finally, mix the dry granules with stearic acid evenly, press into tablets according to the content of the granules to obtain tablet cores, and each tablet is equivalent to containing 400 mg of moxifloxacin;
[0064] Coating: mix two kinds of hypromellose, macrogol 6000, titanium dioxide and red iron oxide evenly, add to purified water and disperse evenly, stir to obtain coating liquid, put the tablet core in the coating pan Inside, control the temperature of the material at 50°C for coating, the weight gain of the coating is 1.0%, stop the coating, and dry to obtain moxiflo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com