Venetoclaxnanocrystal oral solid medicinal composition
A technology of nanocrystals and venetola, applied in the field of medicine, can solve the problems of low bioavailability of final preparations, reduced patient compliance, and large tablet size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
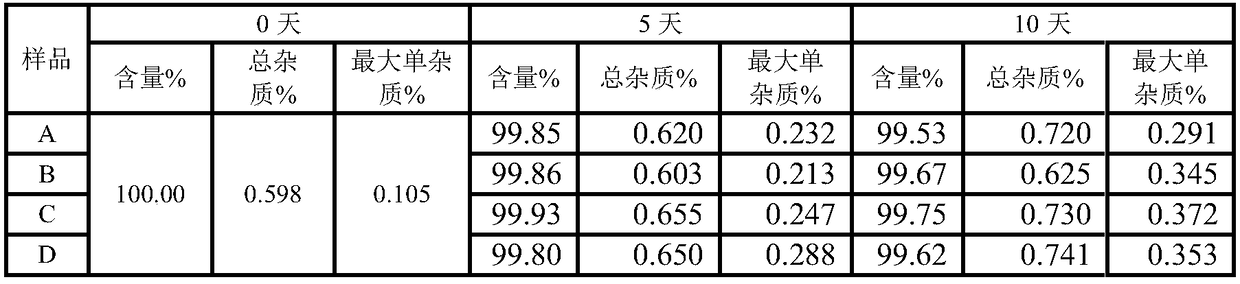
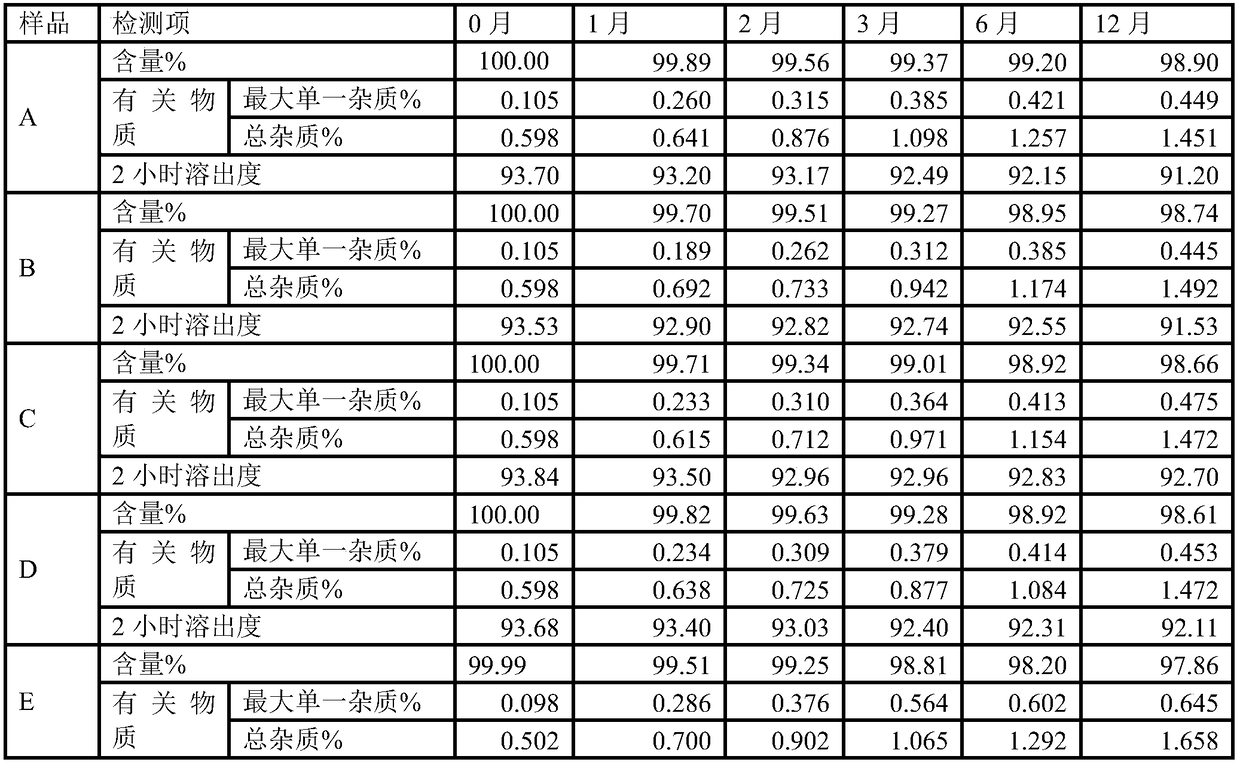
Image
Examples
Embodiment 1
[0102] Example 1 Preparation of 50 mg venetoclax tablet (unit: g)
[0103] prescription:
[0104] .
[0105] The pharmaceutical composition of the venetora nanocrystals is further prepared into tablets by the following steps:
[0106] 1) Take the raw material of venetora and pulverize it, pass through a 200-mesh sieve, and set aside;
[0107] 2) Sodium cholate and hydroxypropyl cellulose were dissolved in purified water successively, with concentrations of 3% and 5%;
[0108] 3) dispersing the venetora crude drug obtained in step 1) in the solution obtained in step 1), with a concentration of 5%;
[0109] 4) Add the suspension obtained in step 3) into a high-pressure homogenizer, and circulate it 10 times under a pressure of 500 bar to obtain a suspension of venetora nanocrystals;
[0110] 5) taking step 4) obtained venetoclax nanocrystal suspension, and spray drying to obtain venetoclax nanocrystal;
[0111] 6) Take the venetoclax nanocrystals obtained in step 5), add ...
Embodiment 2
[0113] Example 2 Preparation of 100 mg venetoclax tablet (unit: g)
[0114] prescription:
[0115] .
[0116] The pharmaceutical composition of the venetora nanocrystals is further prepared into tablets by the following steps:
[0117] 1) Take the raw material of venetora and pulverize it, pass through a 200-mesh sieve, and set aside;
[0118] 2) Sodium cholate and hydroxypropyl cellulose were dissolved in purified water successively, with concentrations of 3% and 5%;
[0119] 3) dispersing the venetora crude drug obtained in step 1) in the solution obtained in step 1), with a concentration of 5%;
[0120] 4) Add the suspension obtained in step 3) into a high-pressure homogenizer, and circulate it 10 times under a pressure of 500 bar to obtain a suspension of venetora nanocrystals;
[0121] 5) taking step 4) obtained venetoclax nanocrystal suspension, and spray drying to obtain venetoclax nanocrystal;
[0122] 6) Take the venetoclax nanocrystals obtained in step 5), add...
Embodiment 3
[0124] Example 3 Preparation of 50 mg venetoclax hard capsules (unit: g)
[0125] prescription:
[0126]
[0127] .
[0128] The pharmaceutical composition of the venetora nanocrystals is further prepared into hard capsules through the following steps:
[0129] 1) Take the raw material of venetora and pulverize it, pass through a 200-mesh sieve, and set aside;
[0130] 2) Sodium cholate and hydroxypropyl cellulose were dissolved in purified water successively, with concentrations of 3% and 5%;
[0131] 3) dispersing the venetora crude drug obtained in step 1) in the solution obtained in step 1), with a concentration of 5%;
[0132] 4) Add the suspension obtained in step 3) into a high-pressure homogenizer, and circulate it 10 times under a pressure of 500 bar to obtain a suspension of venetora nanocrystals;
[0133] 5) taking step 4) obtained venetoclax nanocrystal suspension, and spray drying to obtain venetoclax nanocrystal;
[0134] 6) Take the venetoclax nanocrys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com