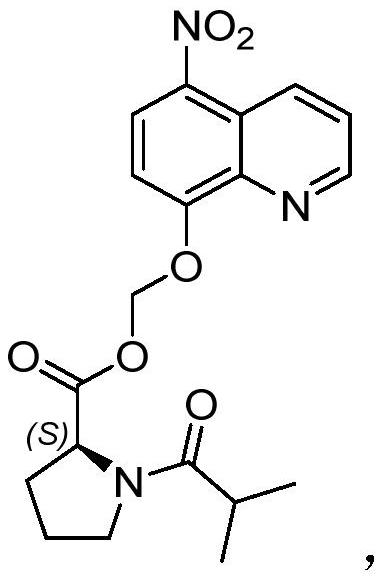
Pharmaceutical composition containing nitroxyquinoline prodrug and its preparation method and application
A nitroxoline and prodrug technology is applied to pharmaceutical compositions containing nitroxoline prodrugs and the fields of preparation and application thereof, and achieves the effects of excellent pharmacokinetic properties, excellent dissolution properties and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
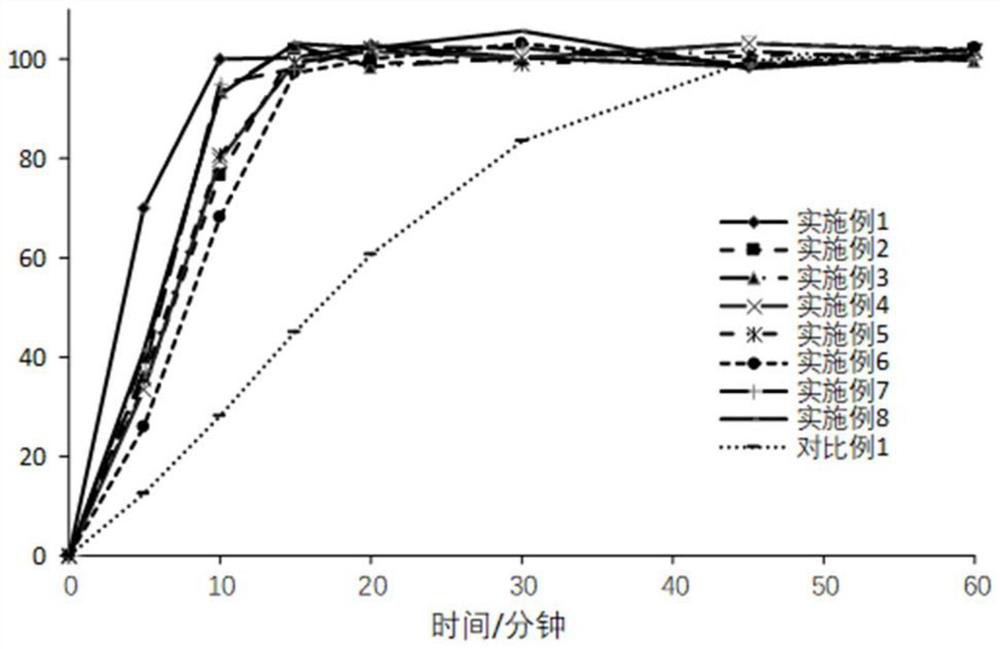
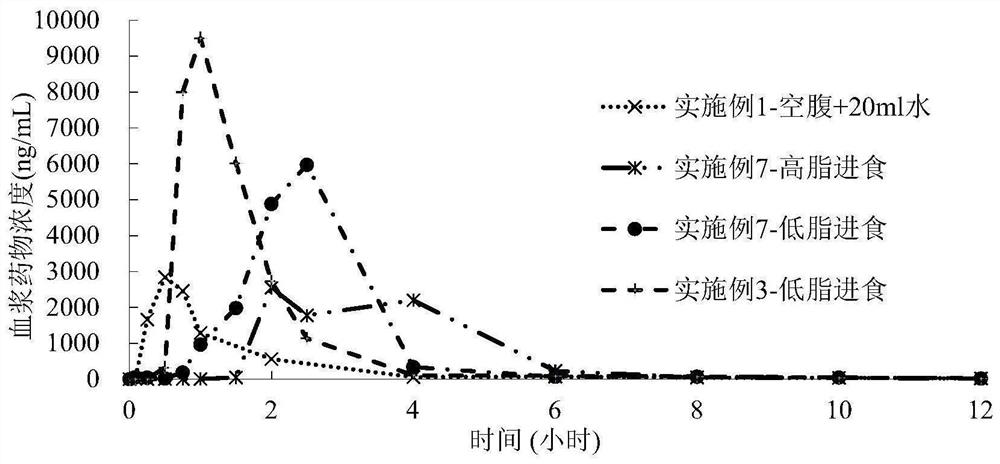
Examples
Embodiment 1
[0085] Made into 500 tablets, each about 160mg.
[0086] Immediate-release tablet preparation method:
[0087] (1) Weigh the API, filler, disintegrant and lubricant of each prescription according to the above table;
[0088] (2) Pass API and other components through a 60-mesh sieve for subsequent use;
[0089] (3) Mix other components in step (2) except the lubricant, add 20-30mL of purified water for wet granulation to prepare soft materials, pass through a 18-mesh sieve for wet granulation, and dry at 60°C until the water content is 1.5-3.5 wt%, use 18-mesh sieve to dry the whole grain, then add lubricant and mix for 10 minutes;
[0090] (4) Use TDP-6 to compress tablets and compress plain tablets;
[0091] (5) BY-300 was used for film coating, the weight gain of the coating was 3%, and the concentration of the coating solution was 15 wt%, to obtain the immediate-release tablet of this embodiment.
Embodiment 2
[0093] Made into 500 tablets, each about 450mg.
[0094] Immediate-release tablet preparation method:
[0095] (1) Weigh the API, filler, binder, disintegrant and lubricant of each prescription according to the above table;
[0096] (2) Preparation of binder (10wt% hydroxypropyl methylcellulose solution): Weigh about 90mL of purified water, slowly add hydroxypropyl methylcellulose, stir while adding, let stand, until completely dissolved ,spare;
[0097] (3) API and other components are passed through a 60-mesh sieve for subsequent use;
[0098] (4) Mix other components in step (3) except the lubricant, add the binder prepared in step (2) and wet granulate to prepare a soft material, pass through a 18-mesh sieve for wet granulation, and dry at 60°C until Moisture content 1.5-3.5wt%, granulate with 18 mesh sieve, then add lubricant and mix for 10 minutes;
[0099] (5) Use ZPS008 to compress the tablet and compress the plain tablet;
[0100] (6) BGB-5F was used for film coa...
Embodiment 3
[0102] Made into 120 tablets, each about 800mg.
[0103] Immediate-release tablet preparation method:
[0104] (1) Weigh the API, filler, disintegrant and lubricant of each prescription according to the above table;
[0105] (2) Pass API and other components through a 60-mesh sieve for subsequent use;
[0106] (3) Mix other components in step (2) except the lubricant, add 25-30mL of purified water for wet granulation to prepare soft materials, pass through a 18-mesh sieve for wet granulation, and dry at 60°C until the water content is 1.5-3.5 wt%, use 18-mesh sieve to dry the whole grain, then add lubricant and mix for 10 minutes;
[0107] (4) Use TDP-6 to compress tablets and compress plain tablets;
[0108] (5) BY-300 was used for film coating, the weight gain of the coating was 3%, and the concentration of the coating solution was 15 wt%, to obtain the immediate-release tablet of this embodiment.
[0109] In addition, according to the same composition, dosage and prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com