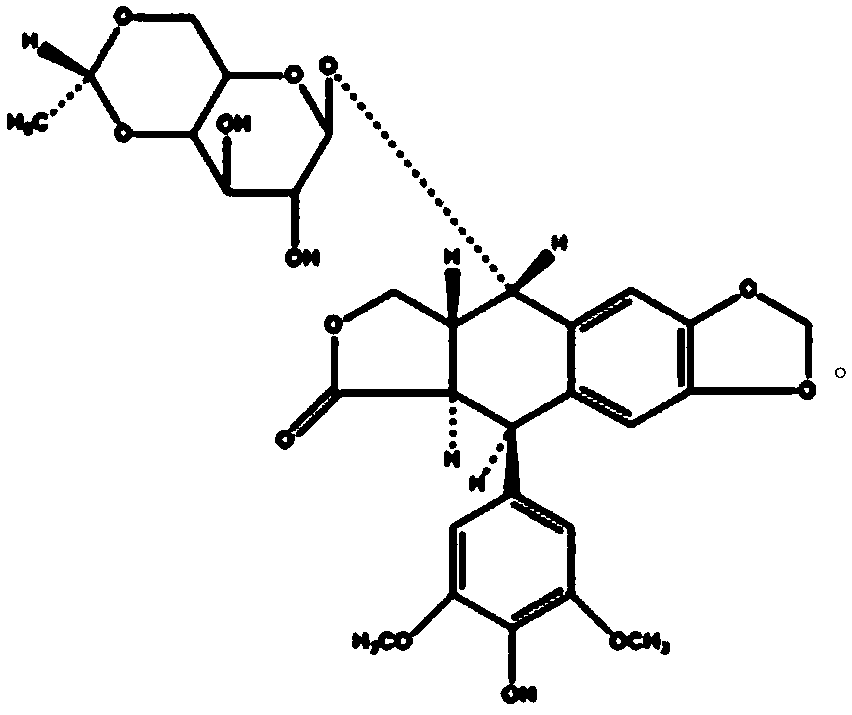
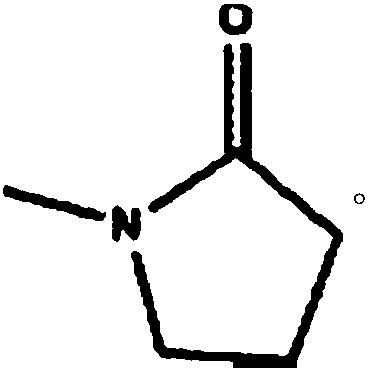
An etoposide solid pharmaceutical composition
A technology of etoposide and poside solid, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 125
[0108] Embodiment 125mg specification etoposide tablet preparation (unit: g)
[0109] prescription:
[0110] Raw materials
Dosage
25g
sodium cholate
25g
Highly substituted hydroxypropyl cellulose
50g
11.6g
Crospovidone
6g
1.2g
1.2g
N-Methylpyrrolidone
Appropriate amount
unit element tablet weight
120mg
Opadry
5% weight gain for plain tablets
Co-made
1000 pieces
[0111] The preparation process is as follows;
[0112] 1) Take the etoposide bulk drug, pulverize it, pass through an 80-mesh sieve, and set aside;
[0113] 2) Take the prescription quantity step 1) obtained etoposide bulk drug, sodium cholate, highly substituted hydroxypropyl cellulose in sequence, dissolve in an appropriate amount of N-methylpyrrolidone, stir, and set aside;
[0114] 3) Take the solution obtained...
Embodiment 250
[0119] Embodiment 250mg specification etoposide tablet preparation (unit: g)
[0120] prescription:
[0121] Raw materials
Dosage
50g
sodium cholate
50g
Highly substituted hydroxypropyl cellulose
100g
23.2g
Crospovidone
12g
2.4g
2.4g
N-Methylpyrrolidone
Appropriate amount
unit element tablet weight
240mg
Opadry
5% weight gain for plain tablets
Co-made
1000 pieces
[0122] The preparation process is as follows;
[0123] 1) Take the etoposide bulk drug, pulverize it, pass through an 80-mesh sieve, and set aside;
[0124] 2) Take the prescription quantity step 1) obtained etoposide bulk drug, sodium cholate, highly substituted hydroxypropyl cellulose in sequence, dissolve in an appropriate amount of N-methylpyrrolidone, stir, and set aside;
[0125] 3) Take the solution obtain...
Embodiment 3100
[0130] Embodiment 3100mg specification etoposide tablet preparation (unit: g)
[0131] prescription:
[0132]
[0133]
[0134] The preparation process is as follows;
[0135] 1) Take the etoposide bulk drug, pulverize it, pass through an 80-mesh sieve, and set aside;
[0136] 2) Take the prescription quantity step 1) obtained etoposide bulk drug, sodium cholate, highly substituted hydroxypropyl cellulose in sequence, dissolve in an appropriate amount of N-methylpyrrolidone, stir, and set aside;
[0137] 3) Take the solution obtained in step 2), and dry it in a vacuum oven to obtain an improved etoposide solid dispersion;
[0138] 4) Take the improved etoposide solid dispersion obtained in step 3), pulverize, pass through a 40-mesh sieve, and set aside;
[0139] 5) Take the pulverized improved etoposide solid dispersion obtained in step 4), add filler microcrystalline cellulose, disintegrating agent crospovidone, glidant colloidal silicon dioxide, lubricant stearin M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com