Ezetimibe oral solid pharmaceutical composition
A technology for ezetimibe and composition, which is applied in the field of ezetimibe oral solid pharmaceutical composition and its preparation field, and can solve the problems of ineffective ezetimibe dissolution rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1: Preparation of ezetimibe tablets with 5 mg specifications (unit: g)
[0108] prescription:
[0109] Raw materials
Dosage
5.0g
5.0g
20.0g
62.0g
Crospovidone
5.0g
2.0g
1.0g
Opadry
5% weight gain for plain tablets
Unit preparation weight (mg)
100mg
Co-made
1000 pieces
[0110] .
[0111] Preparation Process:
[0112] 1) Take the ezetimibe raw material, pulverize it, pass through a 100-mesh sieve, and set aside;
[0113] 2) Take the ezetimibe raw material drug obtained in step 1), the hydrophilic polymer polyvinylpyrrolidone, and the surfactant soybean lecithin are sequentially added to ethanol, stirred, and dissolved to obtain the ezetimibe lipid solution, which is drug adhesive;
[0114] 3) Take the filler microcrystalline cellulose ...
Embodiment 2
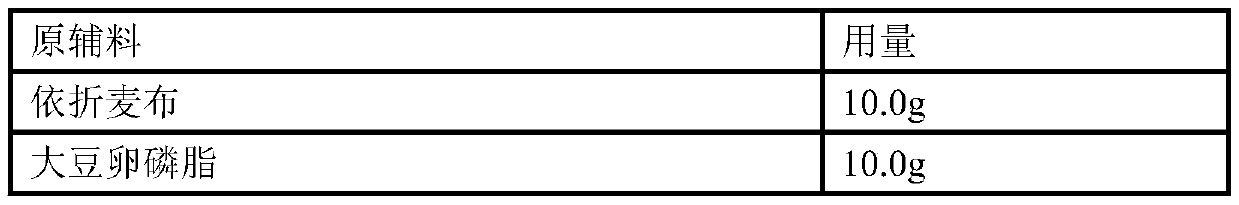
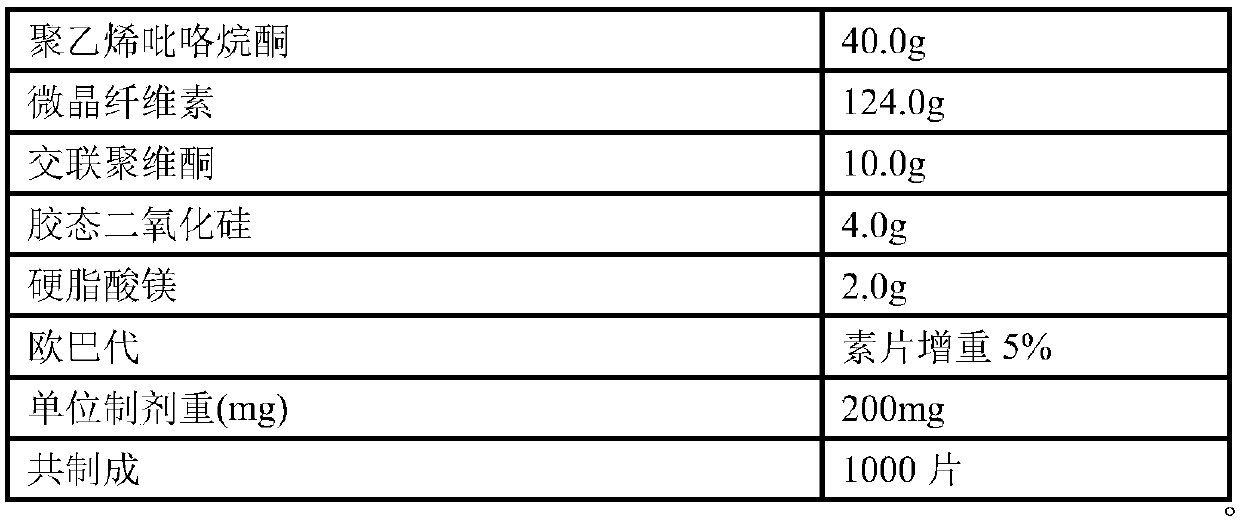
[0118] Example 2: Preparation of 10 mg ezetimibe tablet (unit: g)
[0119] prescription:
[0120] Raw materials
Dosage
10.0g
10.0g
40.0g
124.0g
Crospovidone
10.0g
4.0g
2.0g
Opadry
5% weight gain for plain tablets
Unit preparation weight (mg)
200mg
Co-made
1000 pieces
[0121] .
[0122] Preparation Process:
[0123] 1) Take the ezetimibe raw material, pulverize it, pass through a 100-mesh sieve, and set aside;
[0124] 2) Take the ezetimibe raw material drug obtained in step 1), the hydrophilic polymer polyvinylpyrrolidone, and the surfactant soybean lecithin are sequentially added to ethanol, stirred, and dissolved to obtain the ezetimibe lipid solution, which is drug adhesive;
[0125] 3) Take the filler microcrystalline cellulose and the disinteg...
Embodiment 3
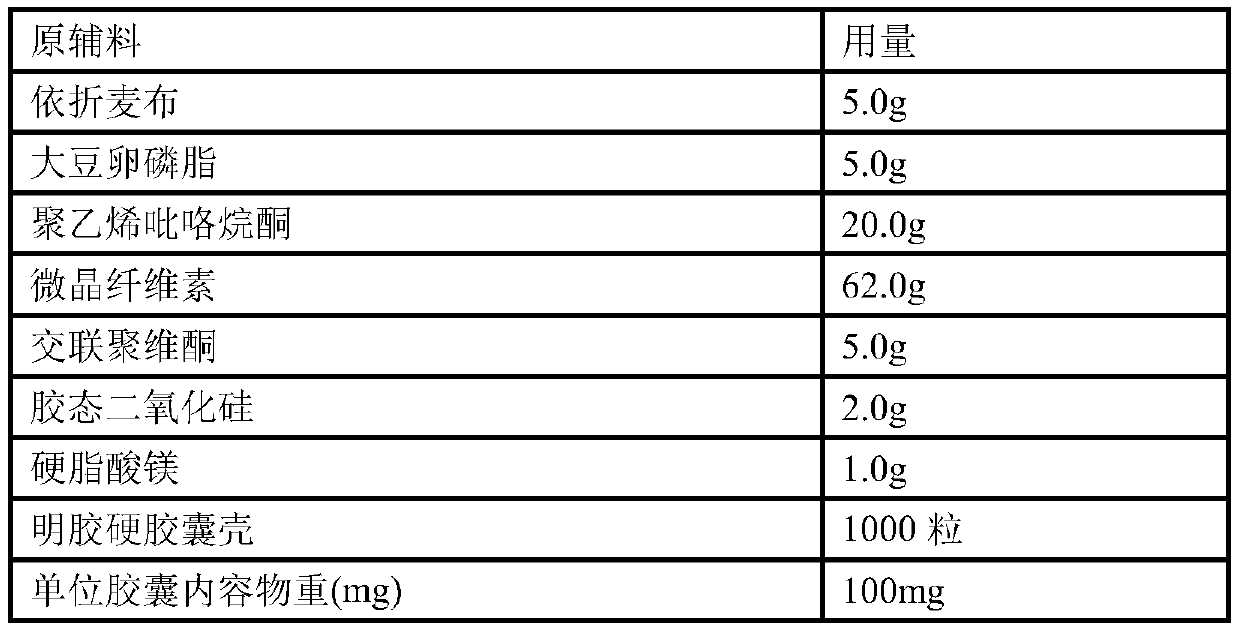
[0129] Example 3: Preparation of 5 mg ezetimibe hard capsules (unit: g)
[0130] prescription:
[0131]
[0132]
[0133] Preparation Process:
[0134] 1) Take the ezetimibe raw material, pulverize it, pass through a 100-mesh sieve, and set aside;
[0135] 2) Take the ezetimibe raw material drug obtained in step 1), the hydrophilic polymer polyvinylpyrrolidone, and the surfactant soybean lecithin are sequentially added to ethanol, stirred, and dissolved to obtain the ezetimibe lipid solution, which is drug adhesive;
[0136] 3) Take the filler microcrystalline cellulose and the disintegrating agent crospovidone, mix them evenly, and add the drug-loaded adhesive obtained in step 2) into a soft material;
[0137] 4) Take the soft material obtained in step 3), granulate it with a 24-mesh sieve, vacuum-dry it, and granulate it with a 40-mesh sieve to obtain dry granules loaded with ezetimibe;
[0138] 5) Take the drug-loaded dry granules obtained in step 4), add a glidan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com