Pharmaceutical composition with improved dissolution properties and apparent solubility and preparation method thereof
A technology for insoluble drugs and solubility, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of low bioavailability, many restrictions, and poor solubility, and achieve easy Industrialized production, wide range of options, stable solubilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0271] In this example, the ursolic acid composition coated with L-grade hypromellose acetate succinate was prepared by drying by rotary evaporation.
[0272] Ursolic acid (5 mg / mL) and L-grade hypromellose acetate succinate (10 mg / mL) were dissolved in ethyl acetate containing 5% methanol and subjected to rotary evaporation. The vacuum degree is 10mbar, the temperature of the water bath is 25°C, and the rotation speed is 200rpm. A film-like composition was obtained.
Embodiment 2
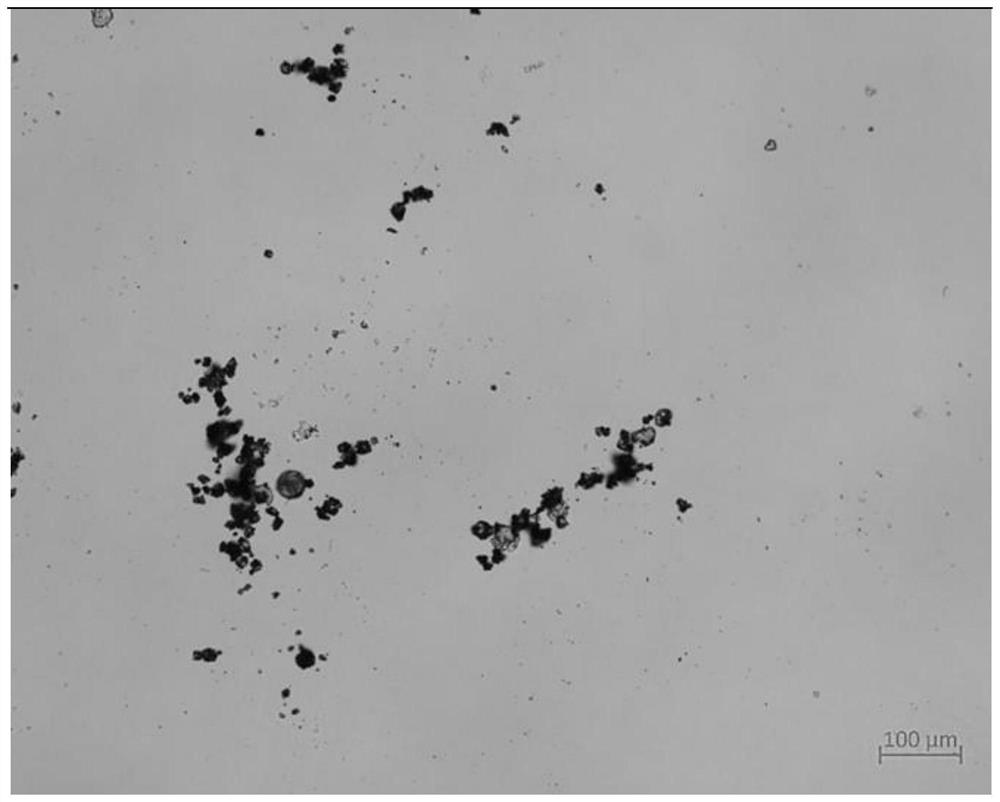
[0274] In this example, the ursolic acid composition coated with L-grade hypromellose acetate succinate was prepared by spray drying.
[0275] Ursolic acid (5 mg / mL) and L-grade hypromellose acetate succinate (10 mg / mL) were dissolved in ethyl acetate containing 5% methanol and spray dried. The inlet temperature was 95°C, the outlet temperature was 60°C, and the injection rate was 10mL / h. Obtain powder composition ( figure 1 ).
Embodiment 3
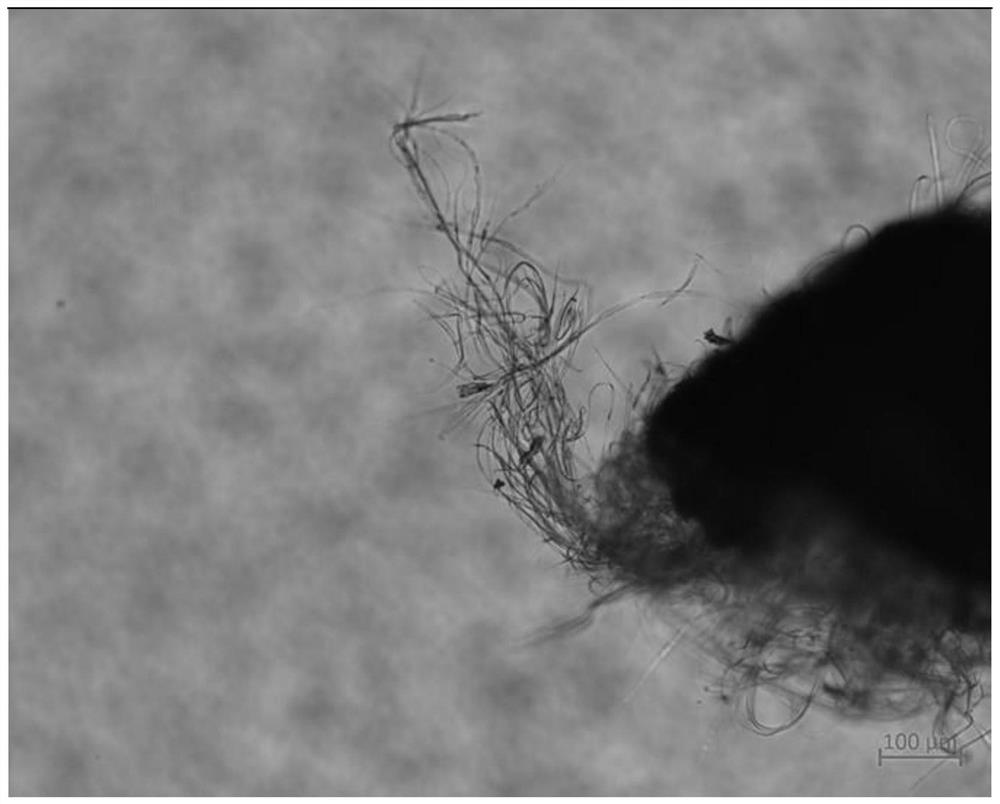
[0277] In this example, an ursolic acid composition coated with L-grade hypromellose acetate succinate was prepared by drying by electrospinning.
[0278] Ursolic acid (5 mg / mL) and L-grade hypromellose acetate succinate (100 mg / mL) were dissolved in ethanol: dichloromethane 1:1 mixed solvent for electrospinning. The positive pressure is 20.0kV, the negative pressure is -1.0kV, and the flow rate is 2mL / h. Obtain fibrous composition ( figure 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com