Pharmaceutical composition containing nitroxoline lysine salt as well as preparation method and application of the pharmaceutical composition
A technology of nitroquinoline lysine salt and composition, which is applied in the field of pharmaceutical composition containing nitroquinoline lysine salt and its preparation and application, and can solve the problem of the absence of nitroquinoline lysine salt. The pharmaceutical composition and other issues, to achieve the effect of good dissolution properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
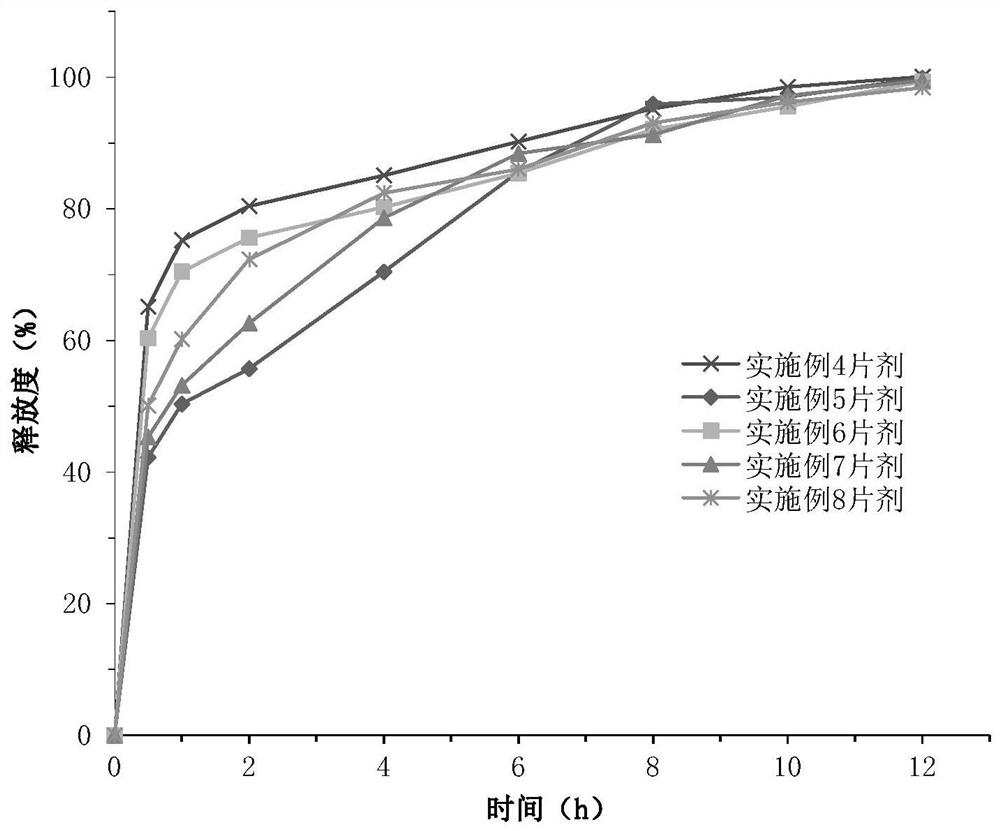
Examples
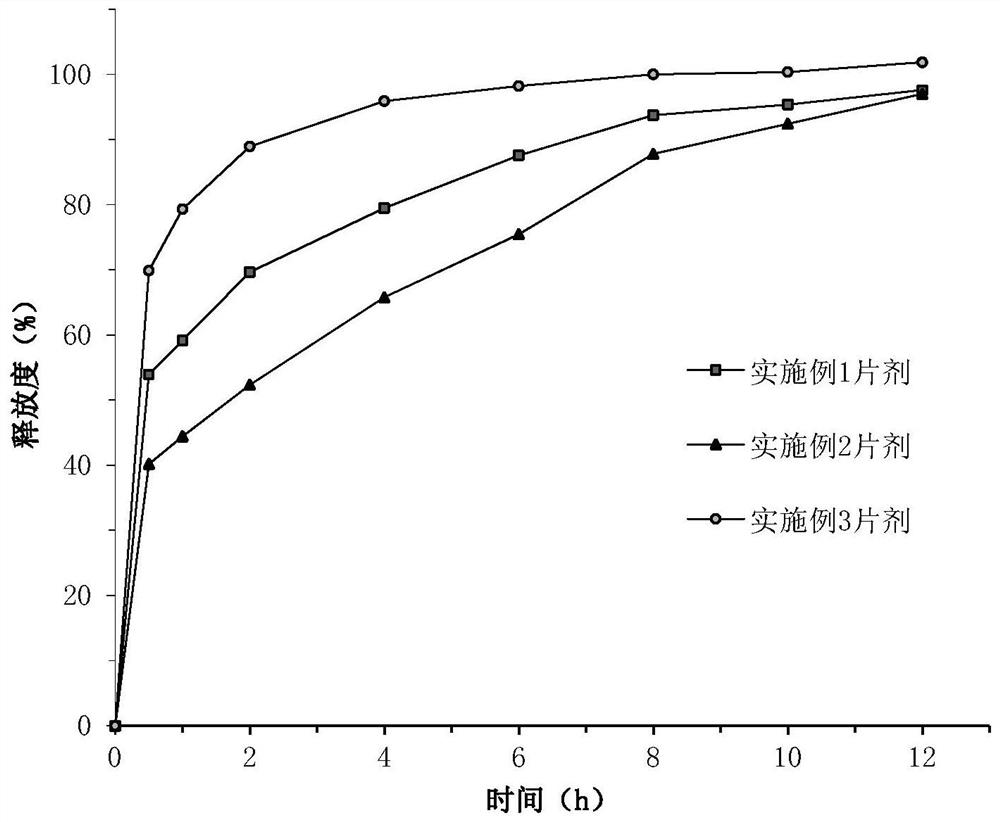
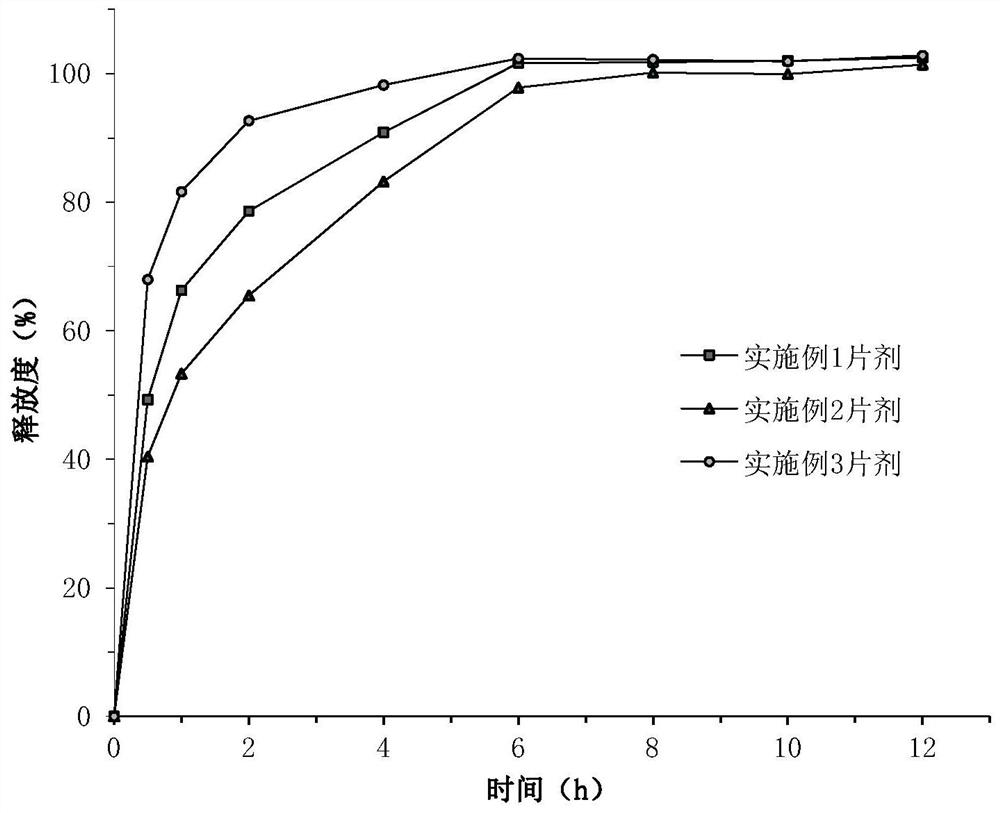
Embodiment 1
[0065] Pass the active pharmaceutical ingredient and each auxiliary material through an 80-mesh sieve for subsequent use.
[0066] Accurately weigh each component respectively according to the above-mentioned first-layer prescription, 5-nitro-8-hydroxyquinoline L-lysine salt monohydrate, microcrystalline cellulose, crospovidone and sodium bicarbonate Mix thoroughly, then prepare soft material with 75wt% ethanol water solution (the consumption of 75wt% ethanol water solution is a conventional dosage, its quality accounts for 10%-30% of the total material quality of the first layer of prescription), pass through a 24 mesh sieve, Dry in a blast drying oven at 60°C for 2 hours, then use a 24-mesh sieve to sieve the granules, then add magnesium stearate and micro-powder silica gel, and mix well to obtain the first layer of granules.
[0067] Accurately weigh each component according to the above-mentioned second-layer prescription, and fully mix 5-nitro-8-hydroxyquinoline L-lysine ...
Embodiment 2
[0070] The first layer of particles and the second layer of particles were prepared according to the method of Example 1.
[0071] Weigh 166.67 mg of the first layer of granules and 333.33 mg of the second layer of granules for use (that is, the mass ratio of the two is 1:2). Pour the second layer of granules into the die ring of the tablet machine, lightly press it flat, and use it as the second layer. Then the first layer of granules is poured into the die ring of the tablet machine, and the first layer is pressed above the second layer to obtain the tablet of Example 2. The diameter of the punch of the tablet press is 11mm, and the pressure is controlled at 60-80N.
Embodiment 3
[0073] The first layer of particles and the second layer of particles were prepared according to the method of Example 1.
[0074] Weigh 333.33 mg of the first layer of granules and 166.67 mg of the second layer of granules for use (that is, the mass ratio of the two is 2:1). Pour the first layer of granules into the die ring of the tablet press, lightly press it flat, as the first layer; then pour the second layer of granules into the die ring of the tablet machine, and press the second layer above the first layer, Obtain the tablet of embodiment 3. The diameter of the punch of the tablet press is 11mm, and the pressure is controlled at 60-80N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com