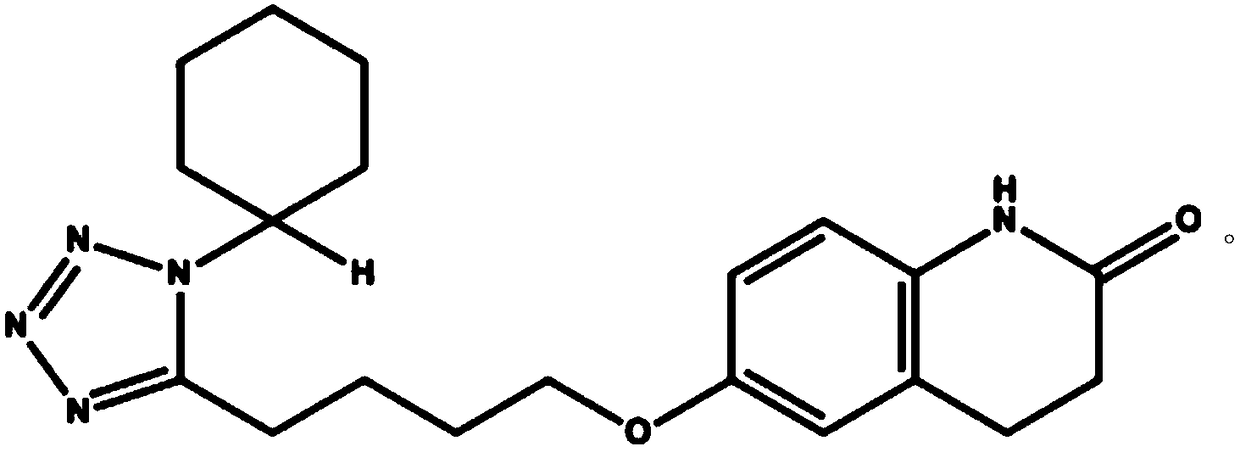
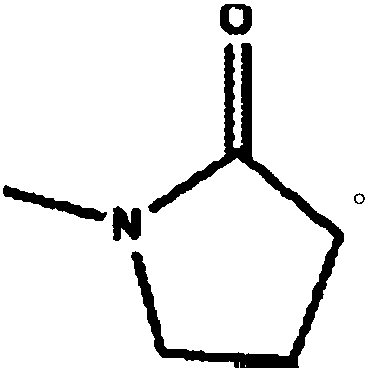
Cilostazol oral solid medicine composition
A technology of cilostazol and a composition is applied in the field of oral solid pharmaceutical composition of cilostazol and its preparation field, and can solve the problems of large preparation volume, small drug-loading amount of solid dispersion, reducing patient compliance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 150
[0111] Embodiment 150mg specification cilostazol tablet preparation (unit: g)
[0112] prescription:
[0113] Raw materials
Dosage
50g
Solutol HS 15
40g
150g
3.6g
Low-substituted hydroxypropyl cellulose
28g
5.6g
2.8g
N-Methylpyrrolidone
Appropriate amount
unit element tablet weight
280mg
Ordinary gastric soluble coating powder Opadry
Co-made
1000 pieces
[0114] The pharmaceutical composition of described cilostazol is further prepared into tablet by the following steps:
[0115] 1) Take the cilostazol crude drug, pulverize it, pass through an 80-mesh sieve, and set aside;
[0116] 2) Take the prescription quantity step 1) successively to obtain the cilostazol raw material drug, Solutol HS 15, polyvinyl alcohol, dissolve in an appropr...
Embodiment 2
[0121] Example 2 Preparation of 100mg Specification Cilostazol Tablets (Unit: g)
[0122] prescription:
[0123]
[0124] .
[0125] The pharmaceutical composition of described cilostazol is further prepared into tablet by the following steps:
[0126] 1) Take the cilostazol crude drug, pulverize it, pass through an 80-mesh sieve, and set aside;
[0127] 2) Take the prescription quantity step 1) successively to obtain the cilostazol raw material drug, Solutol HS 15, polyvinyl alcohol, dissolve in an appropriate amount of N-methylpyrrolidone, stir, and set aside;
[0128] 3) Take the solution obtained in step 2), put it in a vacuum drying oven and dry it to obtain an improved solid dispersion of cilostazol; 4) Take the improved solid dispersion of cilostazol obtained in step 3), pulverize it, and pass through a 40-mesh sieve ,spare;
[0129] 5) Take the pulverized improved cilostazol solid dispersion obtained in step 4), add filler microcrystalline cellulose, disintegr...
Embodiment 3
[0132] Example 3 Preparation of 50 mg specification cilostazol hard capsules (unit: g)
[0133] prescription:
[0134] Raw materials
Dosage
Cilostazol
50g
Solutol HS 15
40g
polyvinyl alcohol
150g
3.6g
Low-substituted hydroxypropyl cellulose
28g
5.6g
2.8g
N-Methylpyrrolidone
Appropriate amount
Unit capsule content weight
280mg
1000 capsules
Co-made
1000 capsules
[0135] The pharmaceutical composition of described cilostazol is further prepared into hard capsules through the following steps:
[0136] 1) Take the cilostazol crude drug, pulverize it, pass through an 80-mesh sieve, and set aside;
[0137] 2) Take the prescription quantity step 1) successively to obtain the cilostazol raw material drug, Solutol HS 15, polyvinyl alcohol, dissolve in an appropriate amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com