Composition with improved dissolution property
A technology of composition and acidic polymer, which is applied in the field of medicine, can solve the problems of research, lack of dissolution test, insufficient physical stability, etc., and achieve the effect of improving dissolution rate and solubility, good stability, and favorable absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Sorafenib: preparation of HPMCP salt
[0047] Sorafenib free base and HPMCP are added into a mixed solvent of methanol and ethyl acetate (1:1 volume ratio) to form a solution, and an ionic bond is formed between the basic sorafenib and the acidic polymer HPMCP.
[0048] 1.2 g of HPMCP was dissolved in a certain volume of solvent by magnetic stirring, then 0.8 g of sorafenib free base was added and allowed to dissolve. Transfer the solution to a 100mL volumetric flask, and add a certain volume of solvent to constant volume.
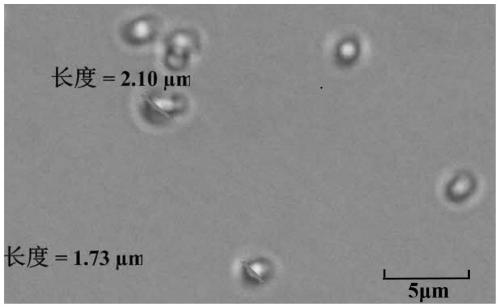
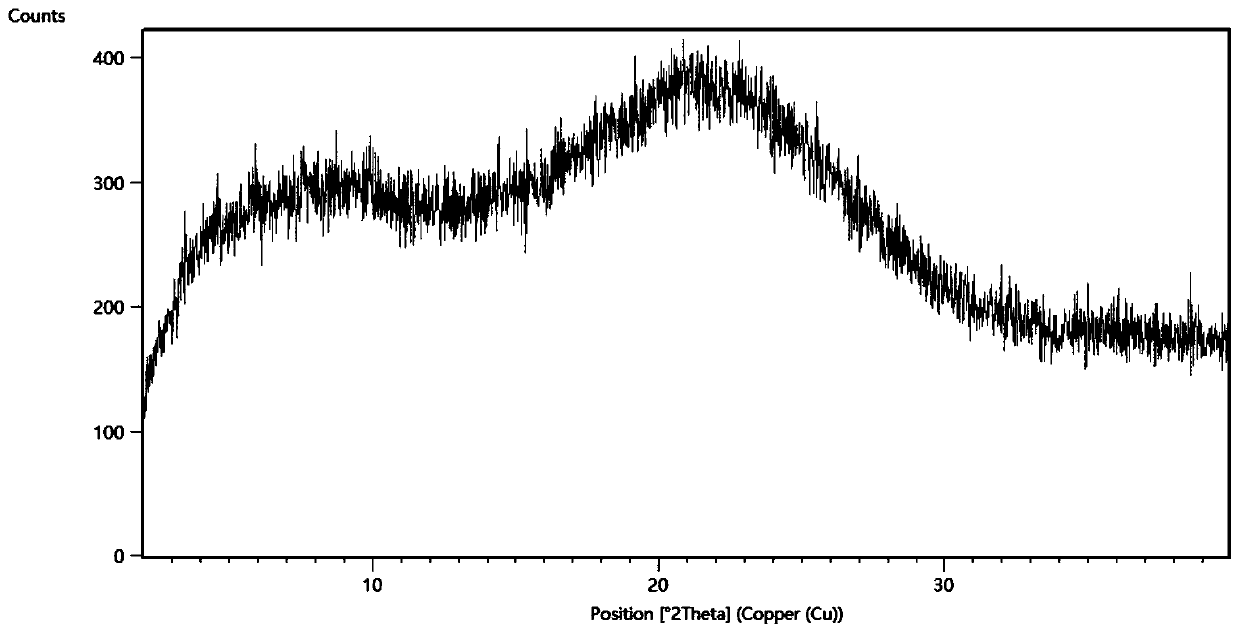
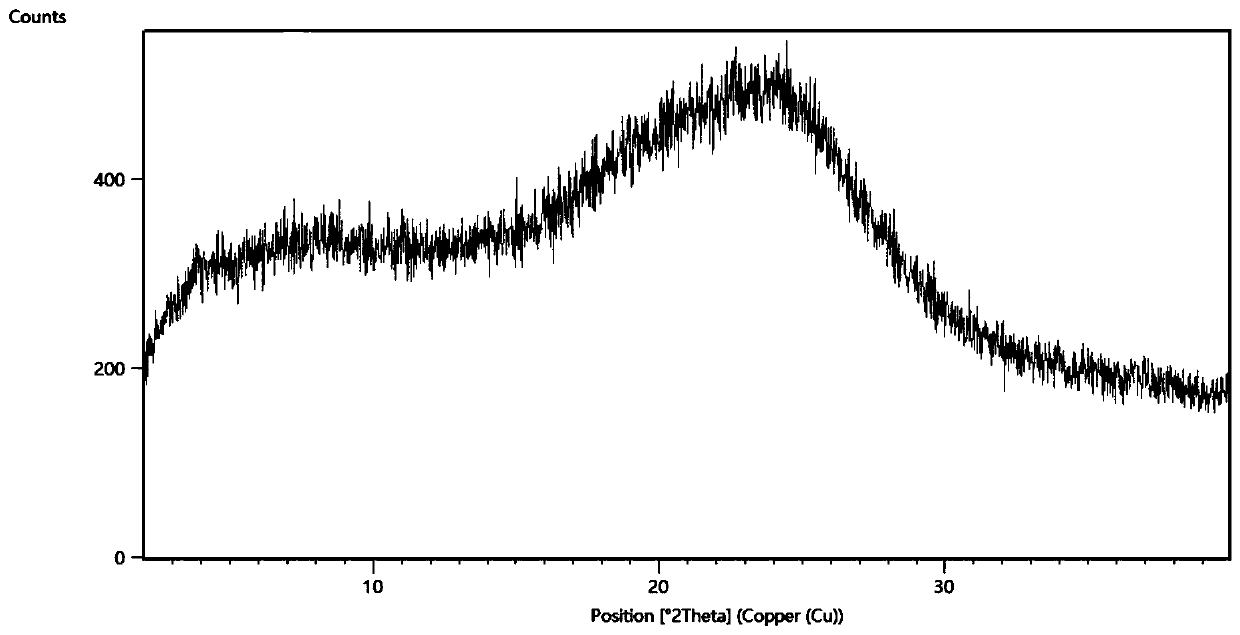
[0049] The obtained Sorafenib containing 40% Sorafenib (mass ratio): HPMCP salt is passed through the Büchi miniature spray dryer B290 ( Labortechnik AG, Switzerland) spray-dried separation. A high-performance cyclone separator is used for separation, and the 50mL blue cap flask can be directly mounted to the cyclone separator for product collection. The parameter settings of the spray drying process are shown in Table 1.
[0050...
Embodiment 2
[0054] Embodiment 2: Gefitinib: preparation of HPMCP salt
[0055] Add gefitinib free base and HPMCP into a mixed solvent of methanol and dichloromethane (1:1 volume ratio) to form a solution, and an ionic bond is formed between the basic gefitinib and the acidic polymer HPMCP.
[0056] 1.2 g of HPMCP was dissolved in a certain volume of solvent by magnetic stirring, then 0.8 g of gefitinib free base was added and allowed to dissolve. Transfer the solution to a 100mL volumetric flask, and add a certain volume of solvent to constant volume.
[0057] The resulting gefitinib containing 40% gefitinib (mass ratio): HPMCP salt was passed through the Büchi miniature spray dryer B290 ( Labortechnik AG, Switzerland) spray-dried separation. A high-performance cyclone separator is used for separation, and the 50mL blue cap flask can be directly mounted to the cyclone separator for product collection. The parameter settings of the spray drying process are shown in Table 2.
[0058] ...
Embodiment 3
[0061] Embodiment 3: Erlotinib: preparation of HPMCP salt
[0062] Erlotinib free base and HPMCP are added into a mixed solvent of methanol and dichloromethane (1:1 volume ratio) to form a solution, and an ionic bond is formed between the basic erlotinib and the acidic polymer HPMCP.
[0063] Dissolve 0.8 g of HPMCP in a volume of solvent by magnetic stirring, then add 1.2 g of erlotinib free base and allow to dissolve. Transfer the solution to a 100mL volumetric flask, and add a certain volume of solvent to constant volume.
[0064] The resulting Erlotinib containing 60% Erlotinib (mass ratio): HPMCP salt was passed through a Büchi miniature spray dryer B290 equipped with an inert cycle B295 ( Labortechnik AG, Switzerland) spray-dried separation. A high-performance cyclone separator is used for separation, and the 50mL blue cap flask can be directly mounted to the cyclone separator for product collection. The parameter settings of the spray drying process are shown in Ta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com