3-((L-valyl)amino)-1-propanesulfonic acid crystal form, preparation method and applications thereof
A technology of valyl and propanesulfonic acid, applied in the direction of organic chemical methods, chemical instruments and methods, preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Preparation of 3-((L-valyl)amino)-1-propanesulfonic acid
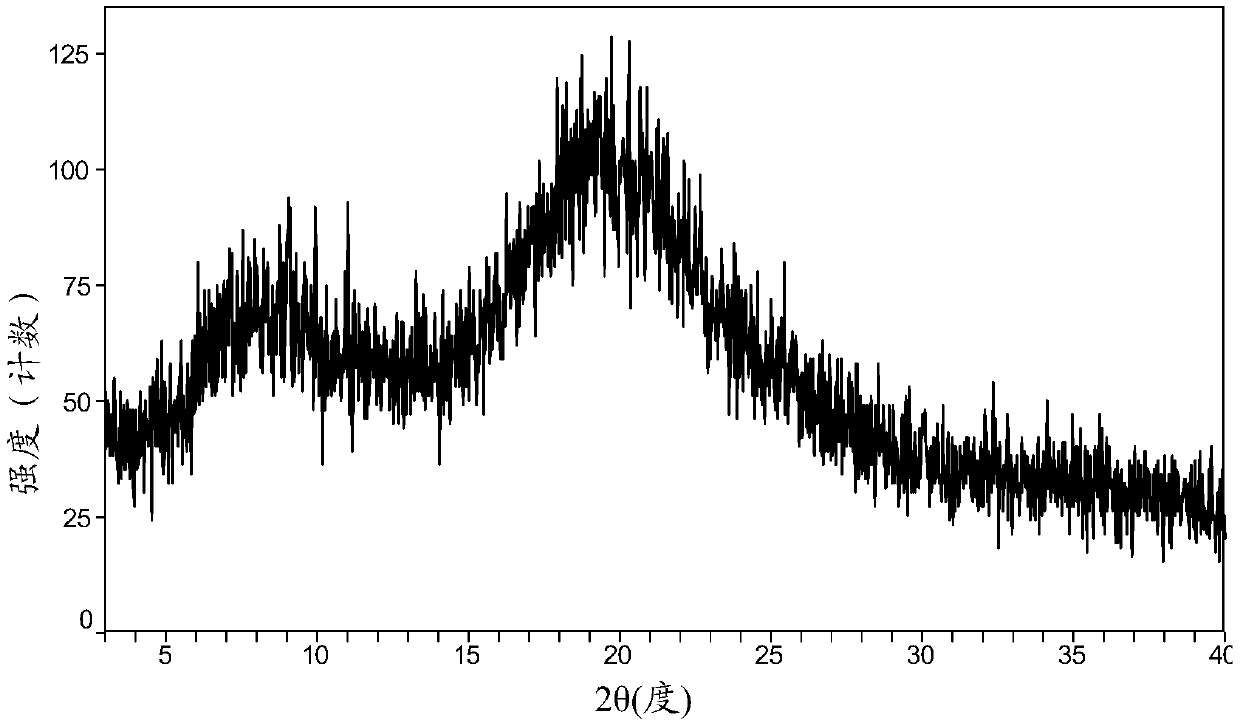
[0125] 1 g of 3-((L-valyl)amino)-1-propanesulfonic acid was dissolved in 5 mL of water, and concentrated under reduced pressure to obtain 1 g of an amorphous solid compound.
[0126] The X-ray powder diffraction (XRD) pattern of this amorphous solid is shown as figure 1 shown.
Embodiment 2
[0128] Preparation of Form I of 3-((L-valyl)amino)-1-propanesulfonic acid
[0129] To 100 mg of the amorphous solid of 3-((L-valyl)amino)-1-propanesulfonic acid described in Example 1 was added a total volume of 5.0 mL of water / ethanol / acetone (1.0 ml / 2.0 ml / 2.0ml) solvent system, wherein the order of adding solvents is to add water (i.e. the first solvent) first, then add ethanol (i.e. the second solvent) and acetone (i.e. the third solvent) successively to obtain the solution, after filtration, the filtrate Placed at room temperature (crystallization temperature) to naturally evaporate to dryness to obtain 95 mg of granular crystals, which is the crystal form I. The yield is 95%, and the purity is greater than 99%.
Embodiment 3
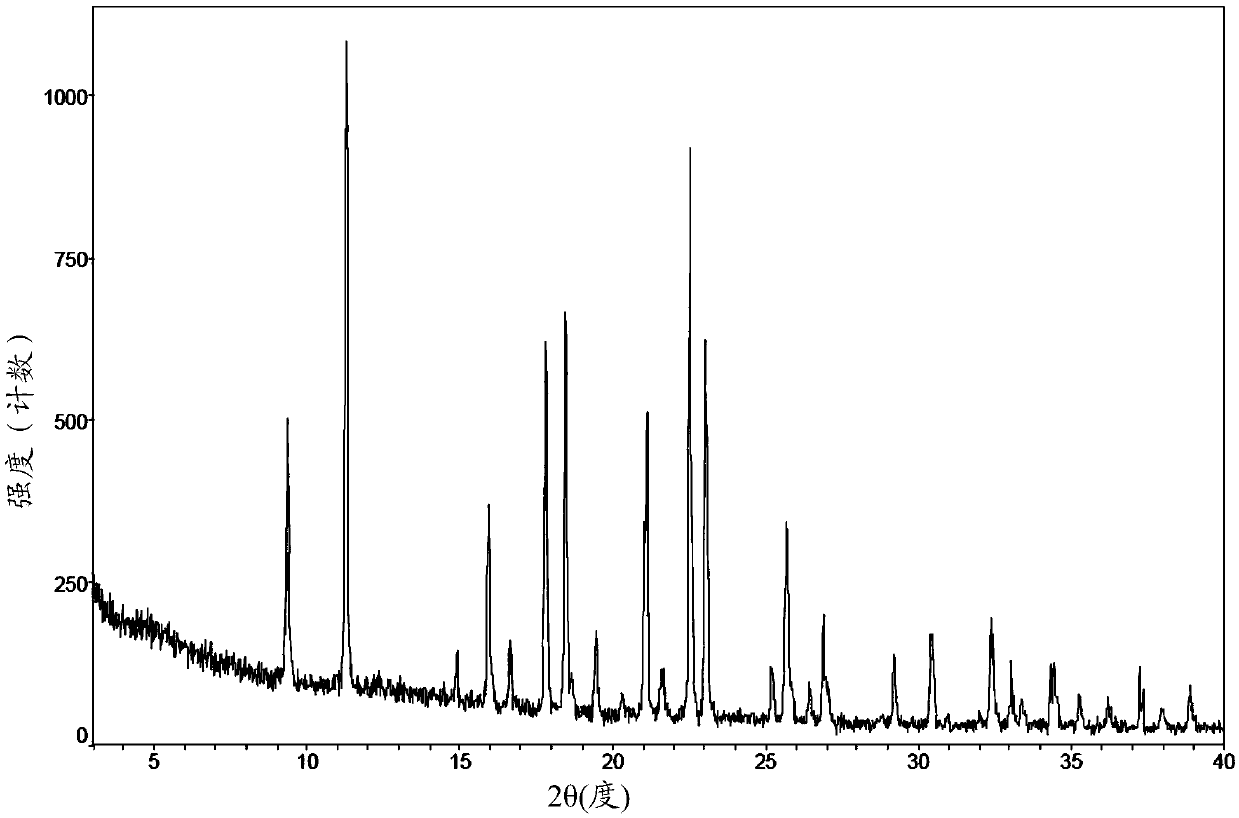
[0131] Determination of the crystalline form I character of the 3-((L-valyl) amino)-1-propanesulfonic acid of the application embodiment 2
[0132] 1. X-ray powder diffraction of the crystal form I
[0133] Adopt the instrument, measuring method and operation condition, parameter as mentioned above to measure. The result is as figure 2 As shown, the specific data is shown in Table 1:
[0134] Table 1 figure 2 X-ray powder diffraction related data
[0135]
[0136]
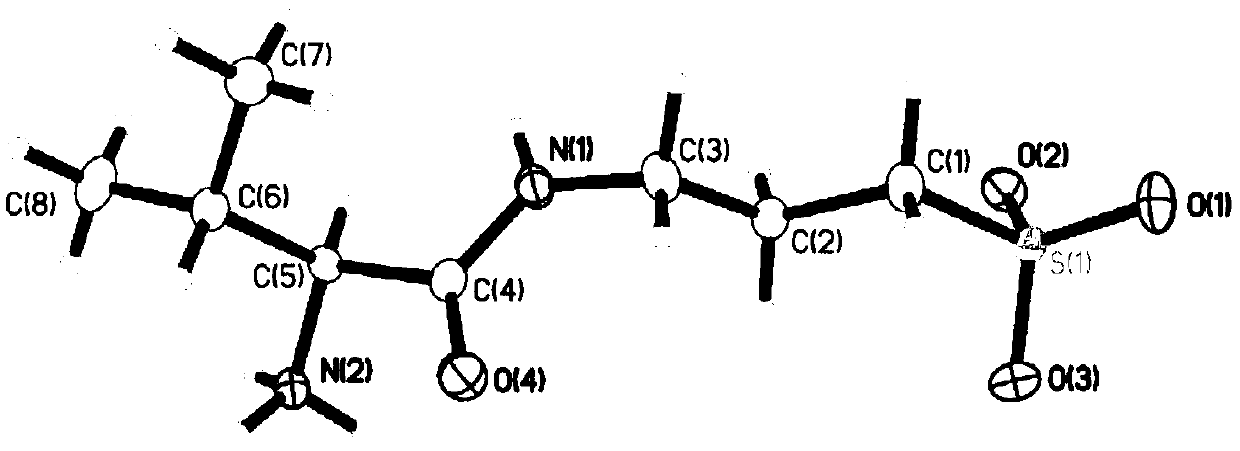
[0137] 2. X-ray single crystal diffraction analysis of the crystal form I
[0138] Adopt the instrument, measuring method and operation condition, parameter as mentioned above to measure. The results are shown below and in Tables 2-3 and Figure 3-4 middle.
[0139] The molecular formula of compound 3-((L-valyl) amino)-1-propanesulfonic acid of the present invention is C 8 h 18 N 2 o 4 S, molecular weight 238.30, density 1.384Mg / m 3 , F(000)=1024. The crystal form I is a white crystal with a size...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com