Method for improving dissolvability of anticoagulant
A technology of additives and granules, applied in pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as solubility drop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] (granulation)
[0155] Under each condition described in Table 1, 1010 g of compound Ia, 2480 g of sieved mannitol (Mannit P, manufactured by TOWA-KASEI Co., Ltd.), 1050 g of pregelatinized starch (PCS PC-10, manufactured by Asahi Kasei Chemicals Corp.) and 267.5 g of crospovidone (Polyplasdone INF-10, manufactured by ISP) with 7 w / v% hydroxypropyl cellulose (HPC-L, manufactured by Nippon Soda Co., Ltd. ) of 2179 mL aqueous solution for fluidized bed granulation. In the fluidized bed granulation, a fluidized bed granulator (FLO-5, manufactured by Freund Corp.) was used
[0156] [Table 1]
[0157]
[0158] (dry).
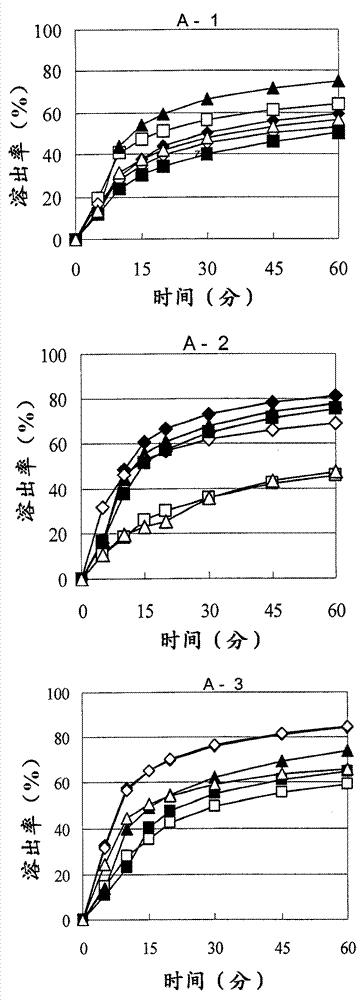
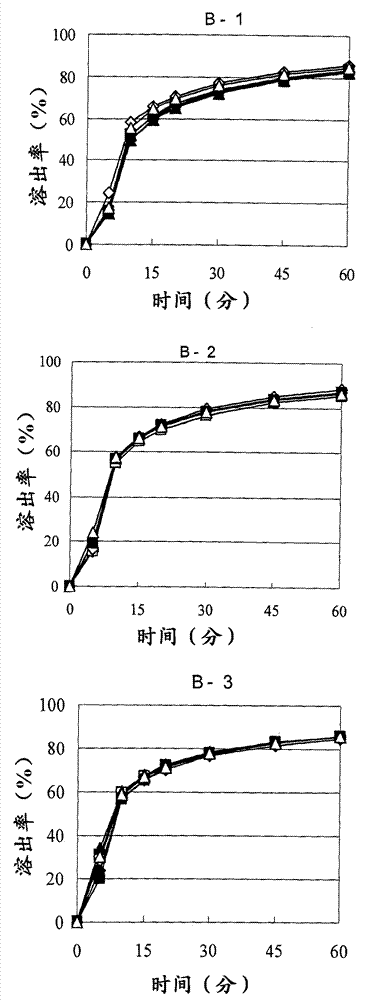
[0159] Next, the granules thus granulated under the respective conditions were dried so that the water content of the granules after drying was 4.0% or more (A-1 and B-1 in Table 2), 2.0% or more and less than 4.0% (A in Table 2). -2 and B-2), or less than 2.0% (A-3 and B-3 in Table 2)
[0160] [Table 2]
[0161]
[0162] (compression).
[0163] 1...
Embodiment 2
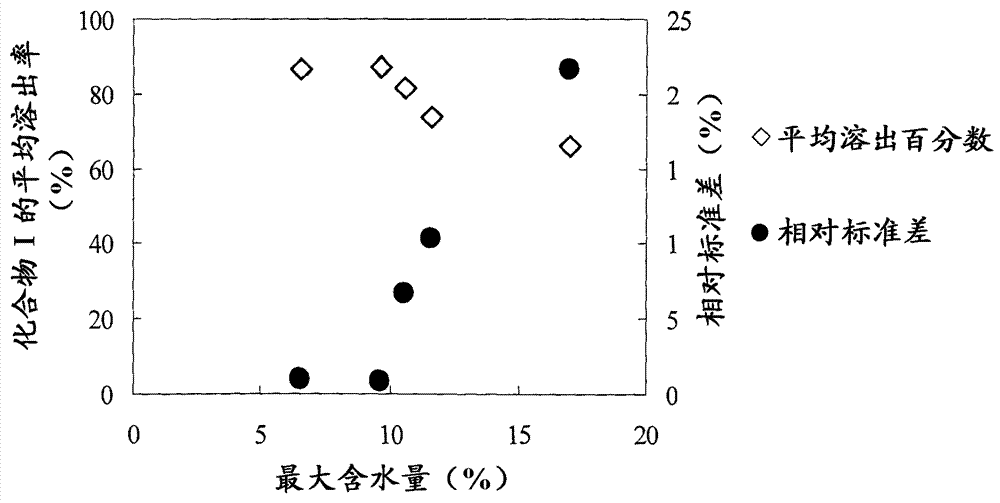
[0175] The dissolution profile of Compound I from each tablet in pH 6.8 phosphate buffer was tested varying the maximum water content of the granules during granulation.
[0176] 7.274 kg of compound Ia, 17.85 kg of sieved mannitol (Mannit P, manufactured by TOWA-KASEI Co., Ltd.), 7.56 kg of pregelatinized starch (PCS PC-10, manufactured by Asahi Kasei Chemicals Corp.) and 1.926 kg Crospovidone (Polyplasdone INF-10, manufactured by ISP) was fluidized with 15.5 L of an aqueous solution containing 7 w / v% hydroxypropylcellulose (HPC-L, manufactured by Nippon Soda Co., Ltd.) Granulate. In fluidized bed granulation, FLO-30 or FLO-30SJ (manufactured by Freund Corp.) is used. The air inlet temperature, liquid spraying speed and spraying air pressure were set to 90 °C, 250 mL / min and 0.25 MPa, respectively. As a result, granules with a maximum water content of 9.6% of the granules during granulation were successfully prepared. Moreover, keeping the granule preparation ratio unchang...
Embodiment 3
[0179] 20.2 kg of compound Ia, 49.6 kg of sieved mannitol (PEARITOL 50C, manufactured by Roquette Corp.), 21 kg of pregelatinized starch (PCS PC-10, manufactured by Asahi Kasei Chemicals Corp.) and 5.35 kg of crospovidone (Polyplasdone INF-10, manufactured by ISP) Fluidized bed granulation was performed with 43.57 kg of an aqueous solution containing 7 w / w% hydroxypropylcellulose (HPC-L, manufactured by Nippon Soda Co., Ltd.). In fluidized bed granulation, WSG-120 (manufactured by Powrex Corp.) was used. The inlet temperature, liquid spraying speed, and air injection volume were set at 90 °C, 700 g / min, and 750 NL / min, respectively, so that the maximum water content (%) of the granules during granulation was below 10%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com