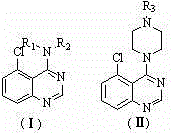
4-N-substituted-5-chloroquinazoline compound and preparation method and application thereof
A technology of chloroquinazoline and compounds, applied in the field of preparation of 4-N-substituted-5-chloroquinazoline compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
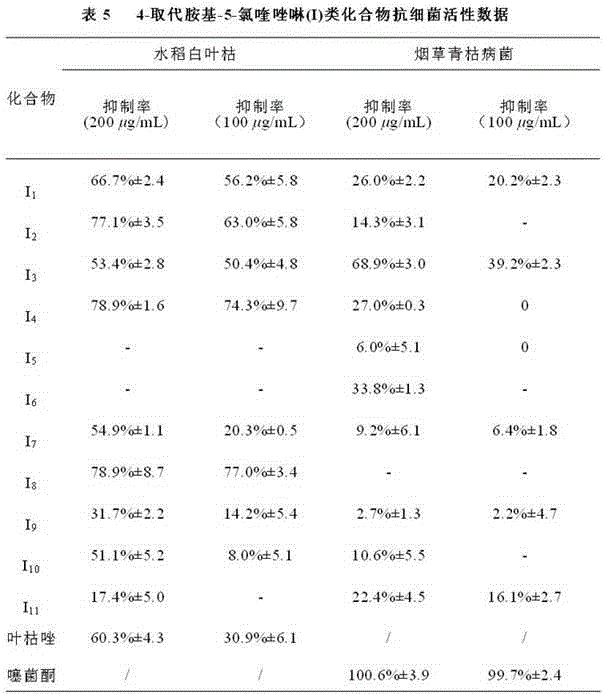
[0089] Example 1, 5-chloro- N -(3-fluorophenethyl)-4-aminoquinazoline (the number of the compound is I 2 )Synthesis
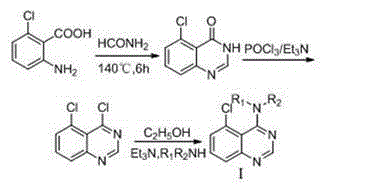
[0090] (1) Synthesis of 5-chloroquinazolin-4(3H)-one
[0091] Into a 25 mL three-neck flask, put 0.86 g (0.005 mol) of 2-amino-6-chlorobenzoic acid, add 3 mL (0.020 mol) of formamide, mix and heat to reflux (140°C) for 6 h, then add 10 mL of water, stir until the temperature is 60°C, then add an appropriate amount of water, cool to room temperature, and filter with suction to obtain 0.61 g of light brown powder, with a yield of 67.8%, m.p. 212 ~ 214°C (literature value 210°C);
[0092] (2) Synthesis of 4,5-dichloroquinazoline
[0093] Into a 50 mL three-neck flask, put 1.00 g (5.42 mmol) of 5-chloroquinazolin-4(3H)-one, and add 13.0 mL POCl 3 and 6 mL of triethylamine, mixed and heated to reflux for 8 h, and distilled under reduced pressure to remove excess POCl 3 , cooled, and added 30 mL CH 2 Cl 2 Dissolve the reaction product, then put the CH 2 Cl...
example 2
[0096] Example two 5-chloro- N -(3,4-dimethoxyphenethyl)-4-aminoquinazoline (the compound number is I 3 )Synthesis
[0097] (1) Synthesis of 5-chloroquinazolin-4(3H)-one
[0098] As in Example 1 (1) synthesis steps and process conditions, the difference is that the molar ratio of 2-amino-6-chlorobenzoic acid to formamide is 1:3.5, the reaction temperature is 120°C, the reaction time is 5h, and the amount of water added per 0.86g 2 - Add 20 mL of water to amino-6-chlorobenzoic acid, add in two times;
[0099] (2) Synthesis of 4,5-dichloroquinazoline
[0100] As in embodiment one (2) synthesis steps and processing conditions, the difference is that 5-chloroquinazoline-4( 3H )-ketone, phosphorus oxychloride and triethylamine mol ratio is 1:12:5.5, and reaction time 9h, 5-chloroquinazoline-4( 3H )-ketone and the mol ratio of dichloromethane is 1:26.5, every 1.00g of 5-chloroquinazoline-4( 3H )-ketone plus 1 mol / L glacial hydrochloric acid 32mL, add in two times;
[0101] (...
example 3
[0103] Example three 5-chloro- N -(2-fluorophenethyl)-4-aminoquinazoline (the compound number is I 4 )Synthesis
[0104] (1) Synthesis of 5-chloroquinazolin-4(3H)-one
[0105] As in Example 1 (1) synthesis steps and process conditions, the difference is that the molar ratio of 2-amino-6-chlorobenzoic acid to formamide is 1:4.5, the reaction temperature is 150°C, the reaction time is 7h, and the amount of water added per 0.86g 2 - Add 24mL of water to amino-6-chlorobenzoic acid, add in two times;
[0106] (2) Synthesis of 4,5-dichloroquinazoline
[0107] As in embodiment one (2) synthesis steps and processing conditions, the difference is that 5-chloroquinazoline-4( 3H )-ketone, phosphorus oxychloride and triethylamine mol ratio is 1:14:6.5, and reaction time 7h, 5-chloroquinazoline-4( 3H )-ketone and the mol ratio of dichloromethane are 1:27.5, every 1.00g of 5-chloroquinazoline-4( 3H )-ketone plus 1 mol / L glacial hydrochloric acid 34mL, add in two times;
[0108] (3) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com