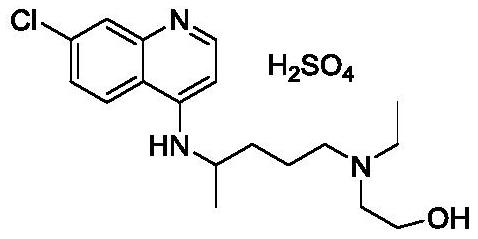
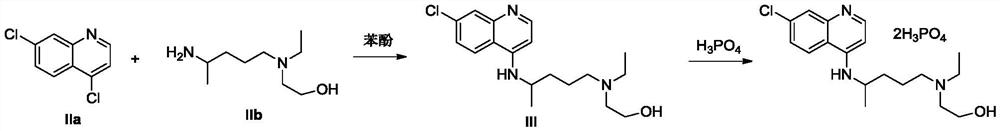
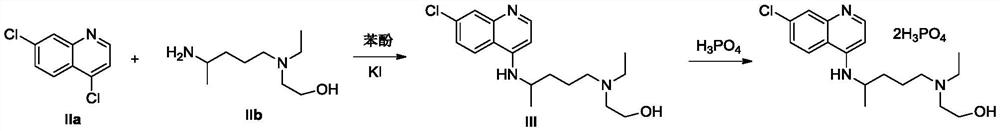
Preparation method of hydroxychloroquine
A technology for hydroxychloroquine and hydroxyl protection, applied in the field of preparation of hydroxychloroquine, can solve the problems of high industrialization cost, low purity of crude product, difficult post-processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of embodiment one hydroxychloroquine
[0035] Under nitrogen protection, add 5-(N-ethyl-N-hydroxyethyl)-2-aminopentane (IIb, 174.3g, 1mol), tetrahydrofuran (872ml), N,N-diisopropyl Ethylethylamine (258.5g, 2mol), cooled to 0-10°C in an ice-water bath, and trimethylchlorosilane (163g, 1.5mol) was added dropwise. / L, 1.5L), continue to stir at the same temperature for 1h, add 4.7-dichloroquinoline (IIa, 174.3g, 1mol), and stir for 3h. Add saturated ammonium chloride solution (1 L), quench the reaction, stir for 30 min, and precipitate a solid, filter, wash with water and n-hexane respectively, and dry to obtain 306 g of hydroxychloroquine as a solid, with a yield of 91.1% (melting point: 89-91.5°C; ESI (+): 336.18; 1HNMR (600MHz, CDCl3) δ8.48 (d, J = 5.4Hz, 1H), 7.93 (d, J = 5.4Hz, 1H), 7.70 (d, J = 9.2Hz, 1H) ,7.34(dd,J=8.8,7.3Hz,1H),6.39(d,J=5.4Hz,1H),4.96(d,J=7.5Hz,1H),3.70(sx,J=6.8Hz,1H) ,3.55(m,2H),2.57(m,5H),2.49(m,2H),1.74–1.62(m,1H),1.65–1.53(m,3H...
Embodiment 2
[0036] The synthesis of embodiment dihydroxychloroquine
[0037] Under nitrogen protection, add 5-(N-ethyl-N-hydroxyethyl)-2-aminopentane (IIb, 348.6g, 2mol), toluene (1.3L), N,N-diiso Propylethylamine (387.8g, 3mol), trimethylchlorosilane (217g, 2mol) was added dropwise at 20-30°C, after the dropwise addition was completed, kept at the same temperature for 1h, LiHMDS tetrahydrofuran solution (1mol / L, 3L ), continue to stir at the same temperature for 1 h, add 4.7-dichloroquinoline (IIa, 174.3 g, 1 mol), and stir for 2 h at the same temperature. Add saturated ammonium chloride solution (1.5L), quench the reaction, stir for 30min, precipitate solid, filter, wash with water and n-hexane respectively, and dry to obtain hydroxychloroquine solid 298.3g, yield 88.8% (melting point: 89~91.0 °C; ESI, [M+1] + :336.2;)
Embodiment 3
[0038] The synthesis of embodiment trihydroxychloroquine
[0039] Under nitrogen protection, add 5-(N-ethyl-N-hydroxyethyl)-2-aminopentane (IIb, 872g, 5mol), tetrahydrofuran (4.4L), N,N-diisopropyl Ethylethylamine (646.3g, 3mol), cooled to 0-5°C in an ice-water bath, and trimethylchlorosilane (542.5g, 5mol) was added dropwise. 1mol / L, 5L), continue to stir at the same temperature for 1h, add 4.7-dichloroquinoline (IIa, 174.3g, 1mol), and stir at 60-65°C for 2h. Add saturated ammonium chloride solution (1 L), quench the reaction, stir for 1 h, and precipitate a solid, filter, wash with water and n-hexane respectively, and dry to obtain 310 g of hydroxychloroquine as a solid, with a yield of 92.3% (melting point: 89.5-91.5°C; ESI,[M+1] + :336.2;)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com