Hydroxychloroquine sulfate pharmaceutical composition and preparation method thereof
A technology of hydroxychloroquine sulfate and composition, which is applied in the field of medicine, can solve problems such as tablet sticking and punching, and achieve the effects of solving tablet sticking, improving adsorption, and quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
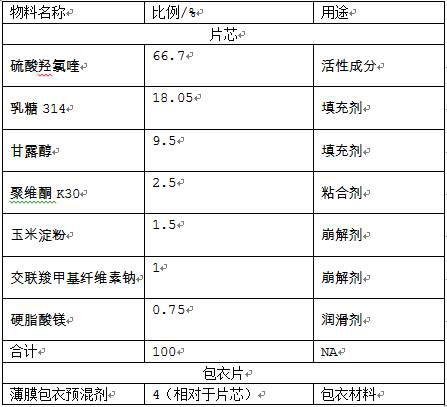
[0026]
[0027] Preparation:
[0028] (1) Add active ingredients, fillers, and binders filtered through a 50-60 mesh screen into a wet mixing granulator for granulation, and then dry and granulate to obtain solid granules;
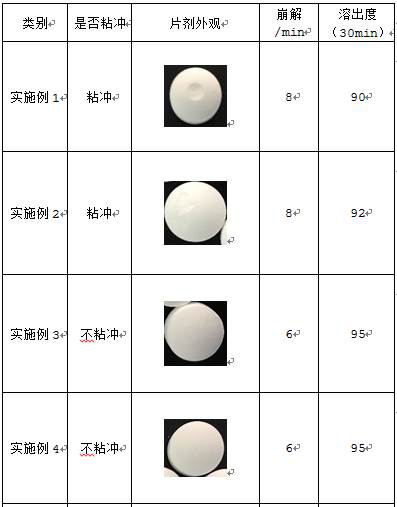
[0029] (2) Add the above-mentioned granules, disintegrating agent, and lubricant into the mixer and granulate again. After tableting, control the humidity between the tablets to ≤40%, control the tablet weight to 290-310mg, and the hardness to 6-10kg to obtain plain tablets ;
[0030] (3) Prepare the film coating premix into a 15% aqueous solution, coat the above-mentioned plain tablets, set the temperature of the coating machine at 50-70°C, atomize at 0.2-0.4MPa, and control the negative pressure of the coating machine at -100pa , Coating weight increase to 2.9-3.1% to stop coating, to obtain the finished product.
Embodiment 2
[0032]
[0033] Preparation:
[0034] (1) Add active ingredients, fillers, and binders filtered through a 50-60 mesh screen into a wet mixing granulator for granulation, and then dry and granulate to obtain solid granules;
[0035] (2) Add the above-mentioned granules, disintegrating agent, and lubricant into the mixer and granulate again. After tableting, control the humidity between the tablets to ≤40%, control the tablet weight to 290-310mg, and the hardness to 6-10kg to obtain plain tablets ;
[0036] (3) Prepare the film coating premix into a 15% aqueous solution, coat the above-mentioned plain tablets, set the temperature of the coating machine at 50-70°C, atomize at 0.2-0.4MPa, and control the negative pressure of the coating machine at -100pa , Coating weight increase to 2.9-3.1% to stop coating, to obtain the finished product.
Embodiment 3
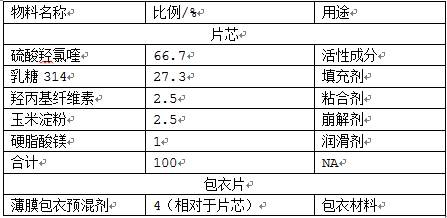
[0038]
[0039] Preparation:
[0040] (1) Add active ingredients, fillers, and binders filtered through a 50-60 mesh screen into a wet mixing granulator for granulation, and then dry and granulate to obtain solid granules;
[0041] (2) Add the above-mentioned granules, disintegrating agent, and lubricant into the mixer and granulate again. After tableting, control the humidity between the tablets to ≤40%, control the tablet weight to 290-310mg, and the hardness to 6-10kg to obtain plain tablets ;
[0042] (3) Prepare the film coating premix into a 15% aqueous solution, coat the above-mentioned plain tablets, set the temperature of the coating machine at 50-70°C, atomize at 0.2-0.4MPa, and control the negative pressure of the coating machine at -100pa , Coating weight increase to 3.1-3.3% to stop coating, to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com