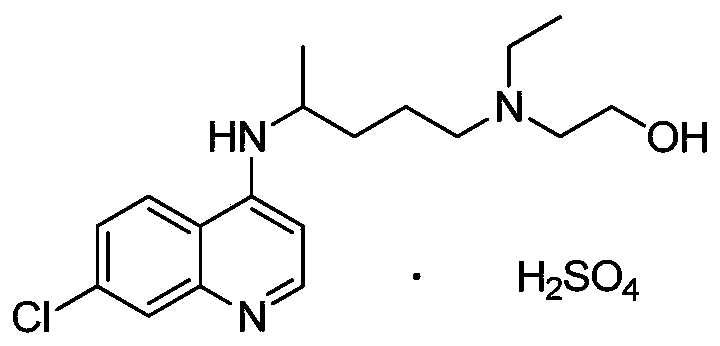
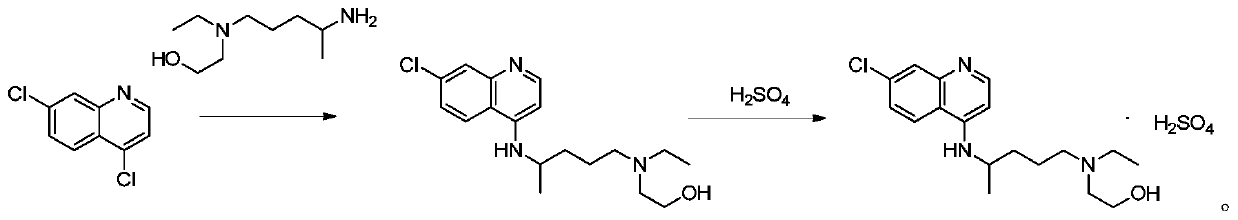
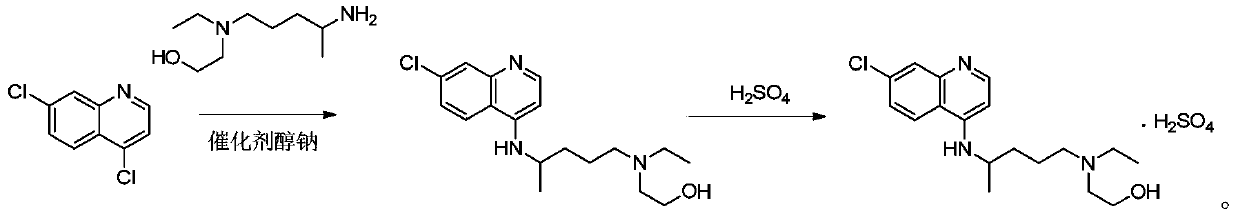
Preparation method of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and hydroxychloroquine, applied in the direction of organic chemistry, etc., can solve the problems of increased impurity content and quantity, unfavorable industrial production, long preparation process time, etc., achieve short reaction time, beneficial to industrial production, and good reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of hydroxychloroquine sulfate:
[0045] (1)
[0046]
[0047] In reaction flask, drop into 4,7-dichloroquinoline (198.0g, 1.0mol), anhydrous KF (116.2g, 2.0mol), hexadecyltrimethylammonium bromide (10.9g, 0.03mol) and acetonitrile (693 mL), nitrogen replacement. The temperature was raised to 80° C., and the reaction was carried out for 2 hours. TLC monitoring showed that the reaction was complete. Add 5-(N-ethyl-N-2-hydroxyethylamine)-2-pentylamine (217.9g, 1.25mol), keep warm and continue to react for 3h. After the reaction is completed, cool down to 30°C, filter with suction, and depressurize the filtrate concentrate. Add 400 mL of purified water to the concentrate, adjust the pH to 10 with 5% sodium hydroxide, precipitate a solid, and filter with suction to obtain crude hydroxychloroquine. Add n-heptane (800mL) to the crude product, heat to reflux, make a slurry, cool to 0°C, heat and crystallize for 2h, filter, and dry at 60°C for 4h to obtain h...
Embodiment 2
[0051] Preparation of hydroxychloroquine:
[0052] The only difference between Example 2 and Example 1 is that the solvent acetonitrile is replaced by the solvent DMF. Hydroxychloroquine (280.1 g) was prepared with a yield of 83.4%, a purity of 99.7%, and a maximum purity of 0.07%.
Embodiment 3
[0054] Preparation of hydroxychloroquine:
[0055] The only difference between Example 3 and Example 2 is that the reaction temperature is 110°C. Hydroxychloroquine (273.8g) was prepared with a yield of 81.5%, a purity of 99.7%, and a maximum of 0.09%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com