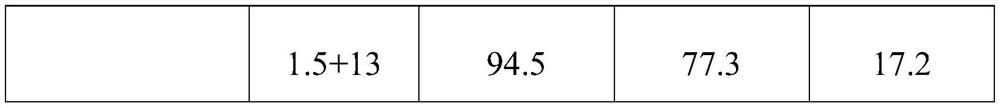
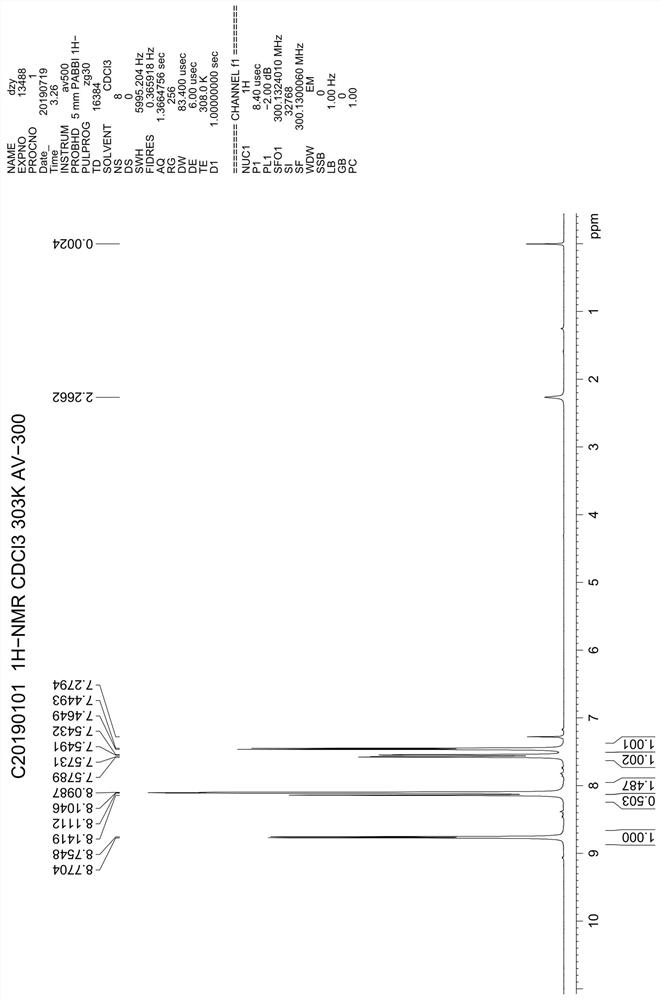
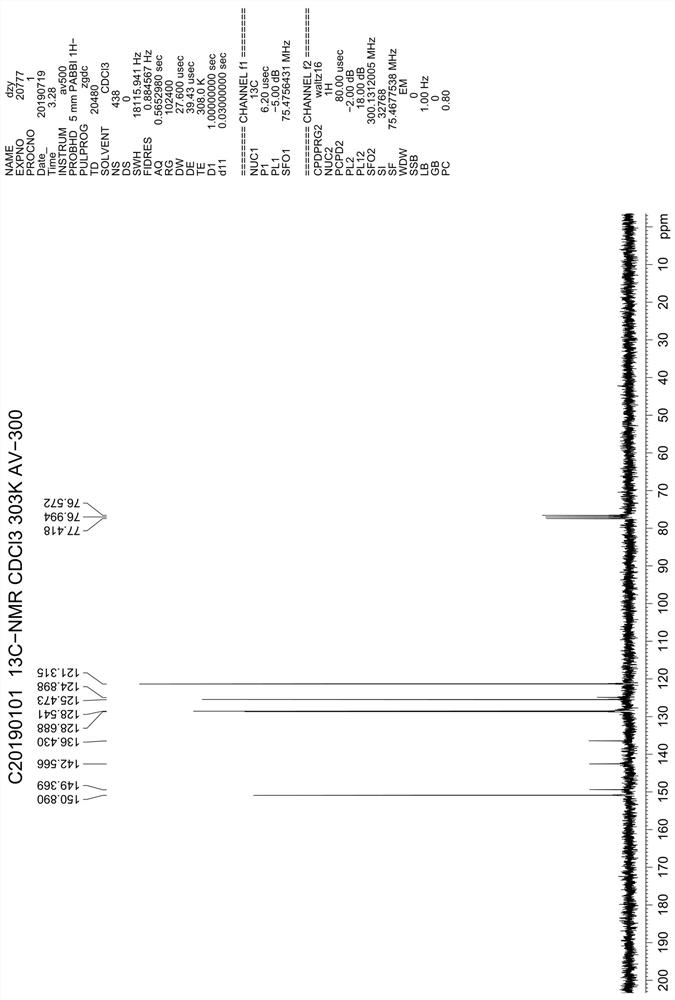
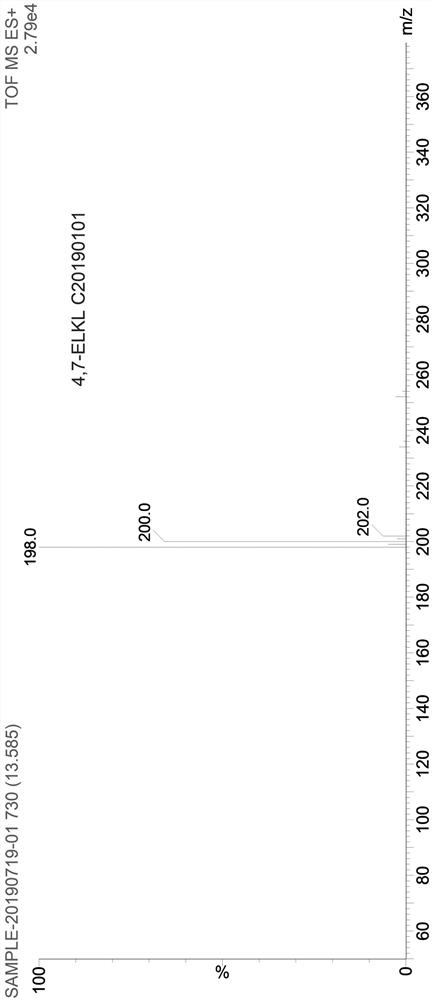
Patents
Literature
37 results about "Dichloroquine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
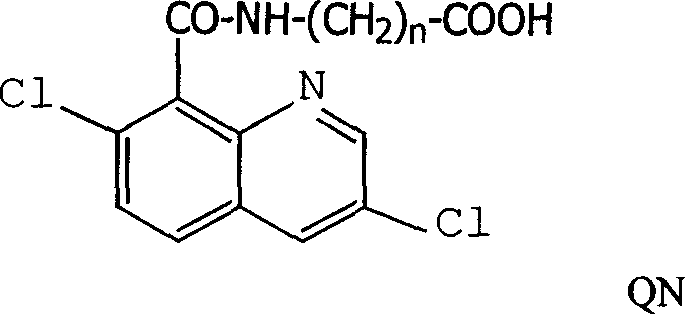
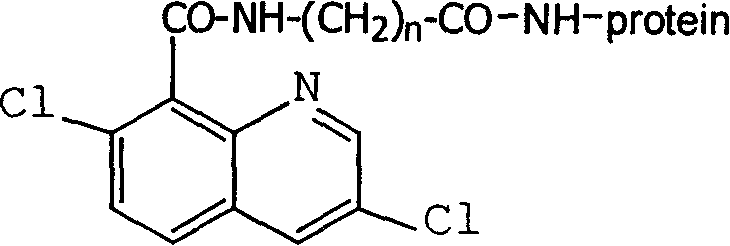
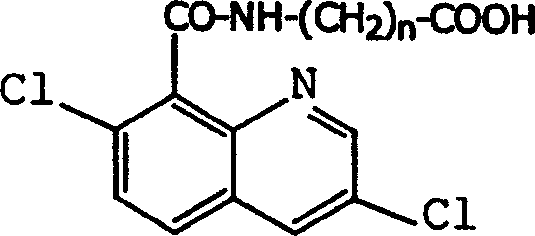
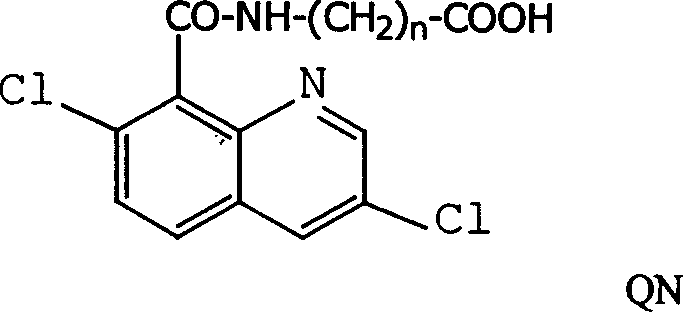
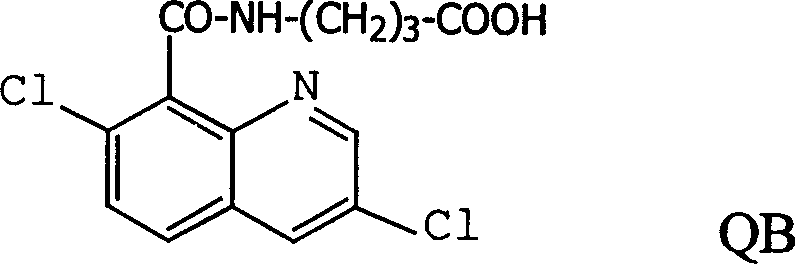
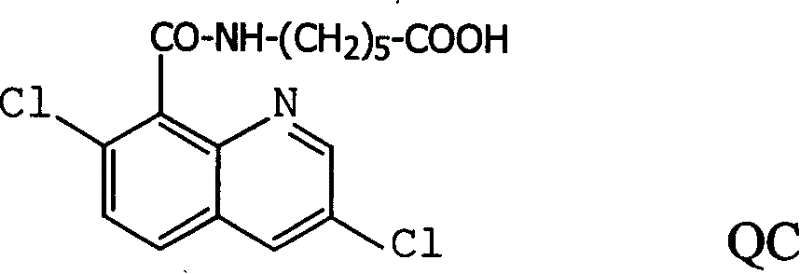
Production method and use for dichloro quinolinic acid artificial hapten, artificial antigen and specific antibody
InactiveCN1569835AEasy to handleFast and accurate analysis and detectionImmunoglobulinsTesting foodQuinolineCarboxylic acid
The invention discloses a process for preparing Quinclorac artificial semiantigen, artificial antigen, specific antibody and use thereof, wherein the preparation comprises, subjecting the dichloroquine (3,7-dichlorine-8-quinoline carboxylic acid) to sulfoxide chlorinated acylation, reacting with reanal and aminocaproic acid, thus obtaining semiantigen 4-(3,7-dichlorine-8-quinolineformyl) reanal or 6-(3,7-dichlorine-8-quinolineformyl) aminocaproic acid (QB or QC).
Owner:ZHEJIANG UNIV
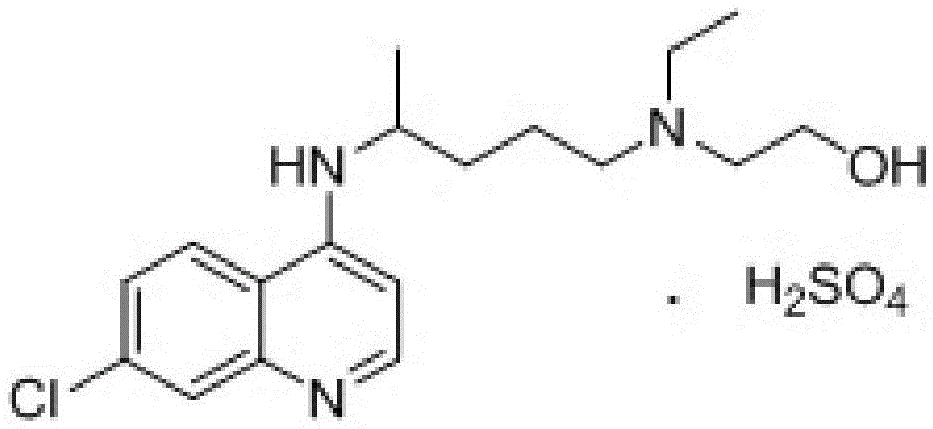
A kind of industrialized preparation method of hydroxychloroquine sulfate
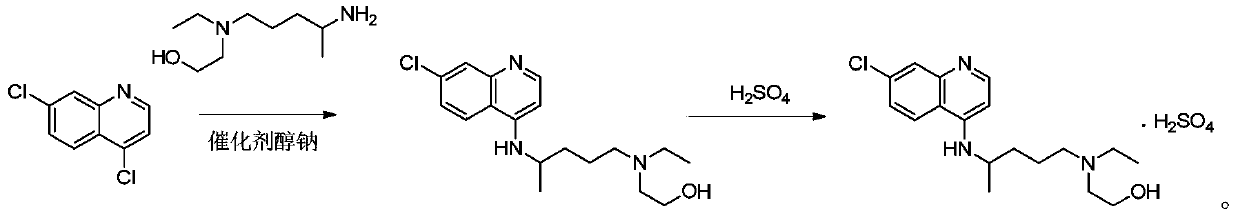
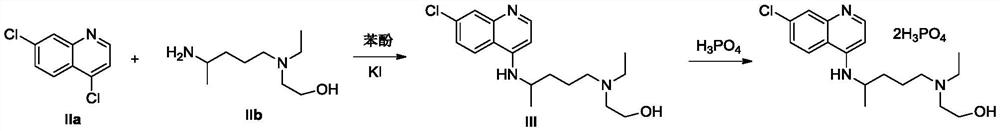
The invention provides an industrial production method for hydroxychloroquine sulfate, which includes the following steps: enabling 4.7-dichloroquinoxaline and 5-(N-ethyl-N-ethoxyl)-2-amino pentane to react under gas shield for 13-24 h at a gradually increased temperature of 120-130 DEG C to obtain hydroxychloroquine; preparing the hydroxychloroquine sulfate after the reaction between the hydroxychloroquine and an alcohol sulfate solution at the temperature of 20-30 DEG C. According to the method, the yield of the obtained crude product of the hydroxychloroquine is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate HPLC is not smaller than 99.5%, the yield of single impurity is not larger than 0.1%, so that requirements of United States Pharmacopeia is met; the novel method is simple in procedure, is environment-friendly and easy in industrial production.
Owner:WUHAN WUYAO PHARMA
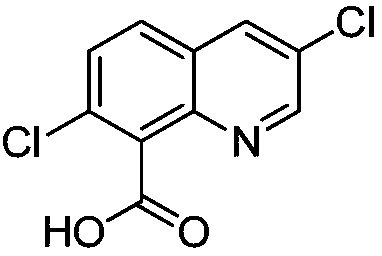
Preparation method of quinclorac
The invention relates to the field of herbicides, and discloses a preparation method of quinclorac, which comprises: 1) adopting an N-hydroxyphthalimide compound and azodiisobutyronitrile as catalysts, adopting oxygen as an oxidizing agent, and oxidizing 7-chloro-8-methylquinoline to obtain 7-chloro-8-quinoline carboxylic acid; and 2) by taking azodiisobutyronitrile as a catalyst, carrying out chlorination reaction on 7-chloro-8-quinoline carboxylic acid and chlorine to obtain 3,7-dichloro-8-quinoline carboxylic acid. By means of the method disclosed by the invention, the generation of a largeamount of waste acid and wastewater in a synthesis process can be reduced, and the product yield of quinclorac can be increased.
Owner:NUTRICHEM LAB CO LTD
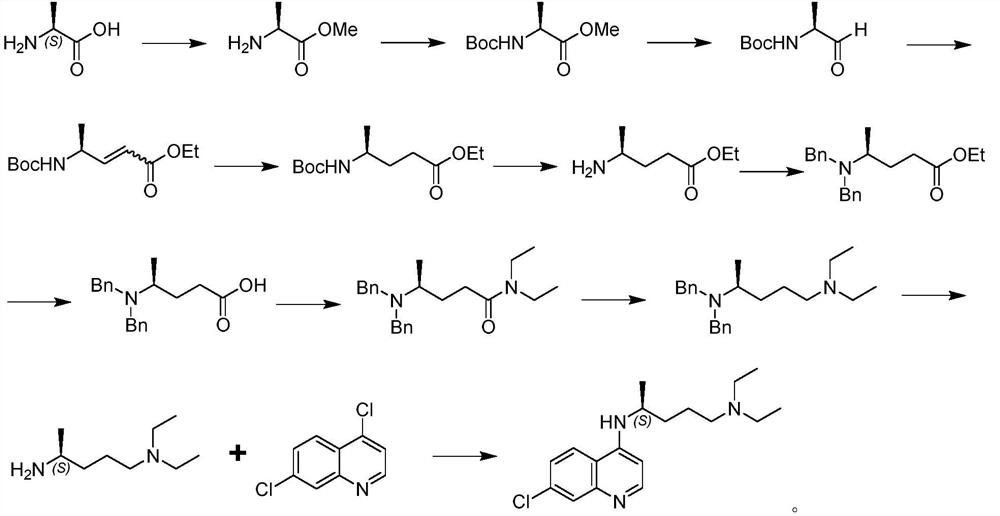
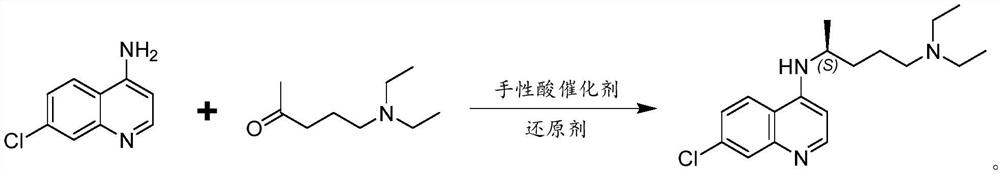
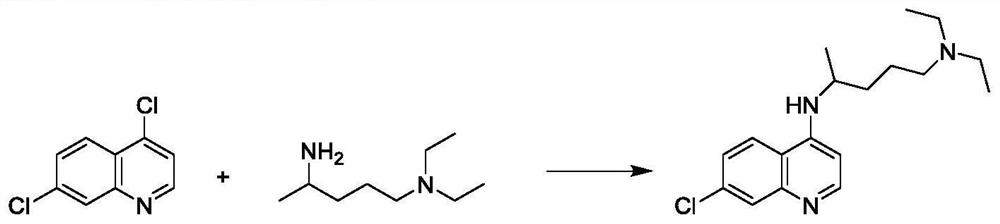
Preparation method of chiral aminochloroquinoline
The invention discloses a preparation method of chiral aminochloroquinoline, which comprises the following steps: splitting a chiral side chain, preparing enantiomer salt, splitting the side chain andchiral acid crystals to obtain chiral acid salt, neutralizing the chiral acid salt to obtain free basic groups, and reacting the free basic groups with 4, 7-dichloroquinoline to obtain chiral aminochloroquinoline. The yield of the chiral aminochloroquinoline obtained by the method is high, and the purity reaches 99.7%.
Owner:珠海润都制药股份有限公司 +1
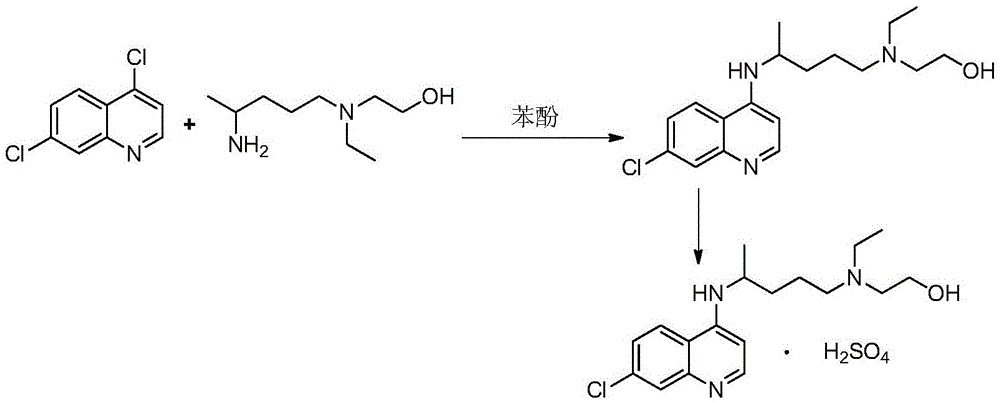
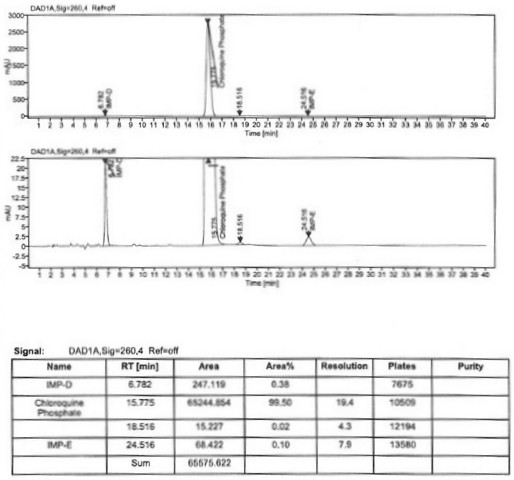
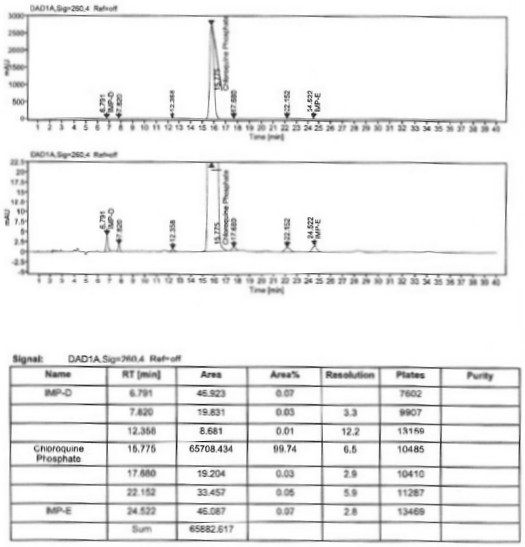
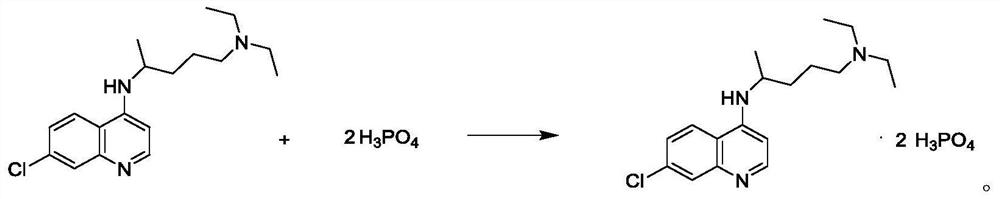
Preparation process of chloroquine phosphate
ActiveCN111662229AReduce usageHigh purityOrganic chemistryAgainst vector-borne diseasesO-Phosphoric AcidPtru catalyst
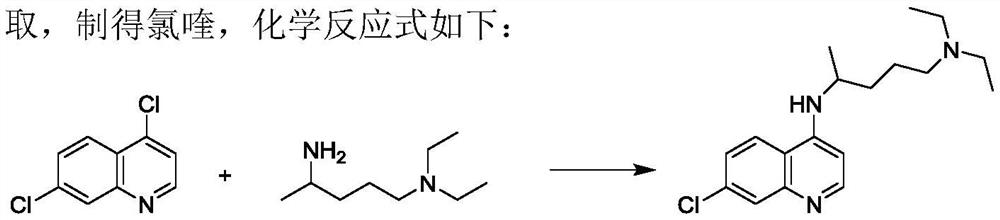
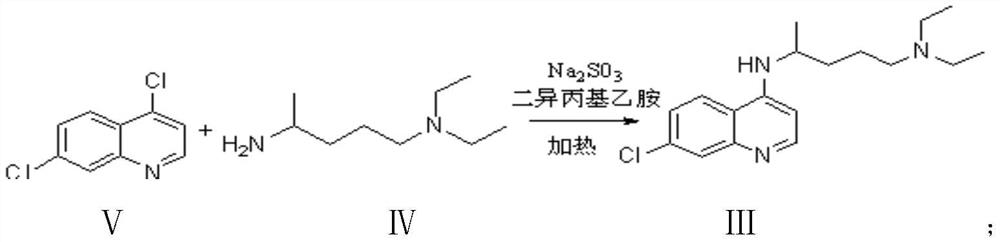
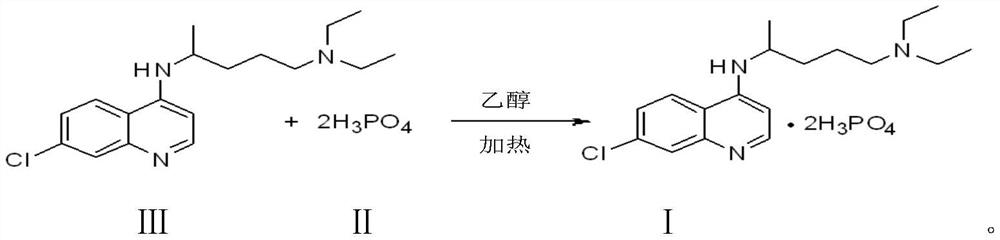
The invention discloses a preparation process of chloroquine phosphate. The preparation process of the chloroquine phosphate comprises the following steps of: (1) taking 4, 7-dichloroquinoline as an initial raw material, carrying out condensation reaction with 2-amino-5-diethylaminopentane, and carrying out alkalization extraction to obtain chloroquine; and (2) salifying the chloroquine obtained in the step (1) with phosphoric acid to obtain chloroquine phosphate. The invention provides a preparation process of chloroquine phosphate. In the preparation process, the use of organic solvents suchas benzene and other catalysts such as phenol is avoided, and a condensation crystallization impurity removal step is introduced, so that the product purity is high, the individual impurity is less than 0.1%, the green chemical requirements and pharmacopeia standards are met, the operation is simple and convenient, the process is simplified, the production efficiency is improved, and the method is suitable for industrial production.
Owner:JINGHUA PHARMA GRP NANTONG
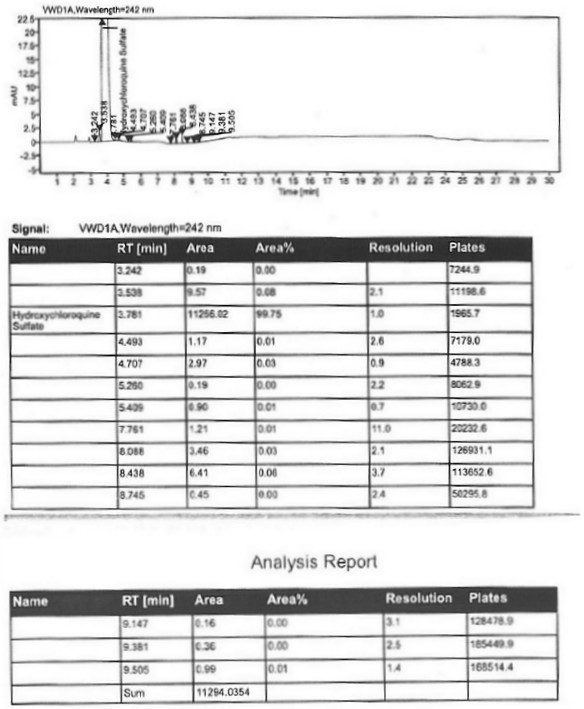
Hydroxychloroquine sulfate and preparation method thereof
PendingCN113185459AGood pollution effectSpeed up the reaction processOrganic chemistryHydroxychloroquinePtru catalyst
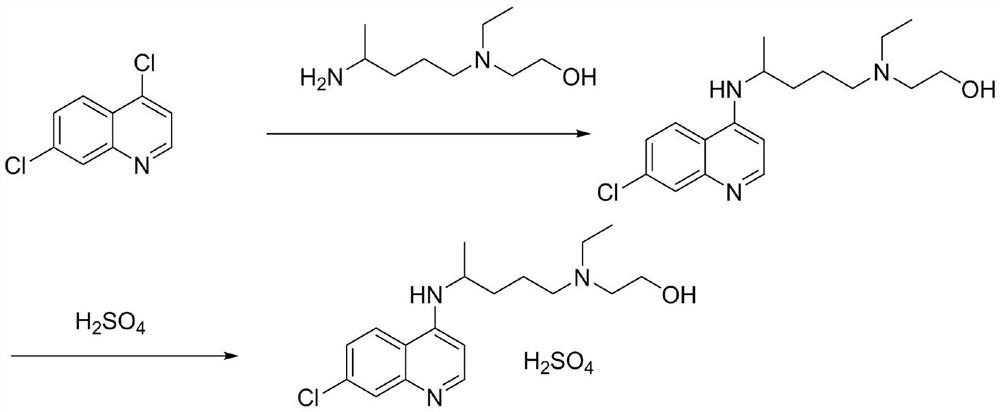
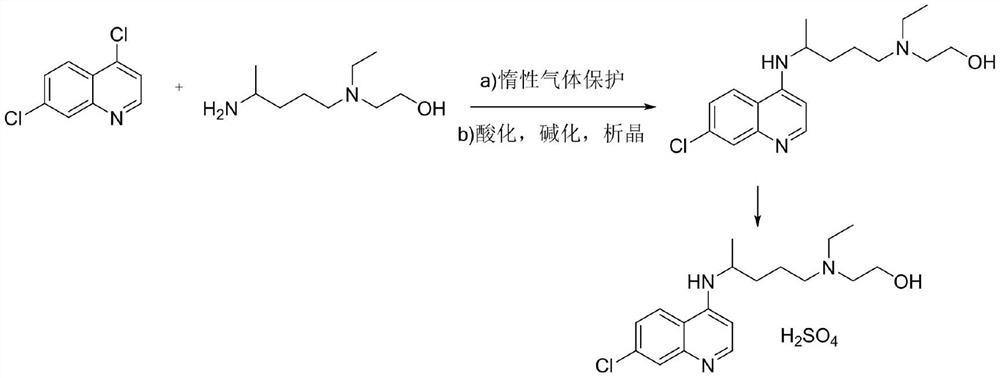
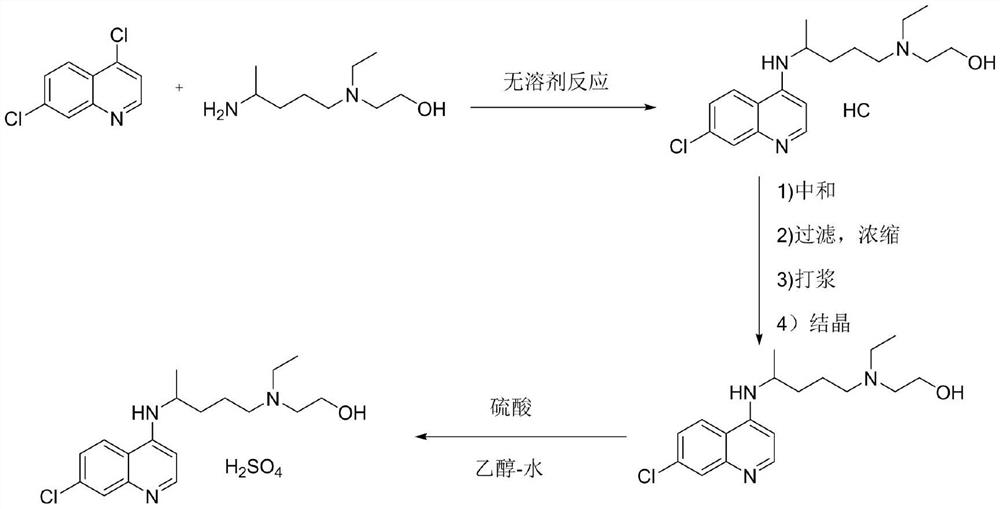
The invention discloses hydroxychloroquine sulfate and a preparation method thereof, and relates to the technical field of medicinal chemistry. The preparation method comprises the steps of mixing 4, 7-dichloroquinoline with a hydroxychloroquine side chain, carrying out heating condensation in the presence of an organic base catalyst, adding water and liquid, and carrying out cooling crystallization to obtain hydroxychloroquine; and dissolving hydroxychloroquine in an ethyl acetate and ethanol aqueous solution, heating, dissolving and clarifying, dropwise adding concentrated sulfuric acid, cooling, crystallizing, filtering and drying to obtain hydroxychloroquine sulfate. The method has the beneficial effects that a solvent-free reaction is used, and a catalyst is added, so that high pollution is avoided, the reaction process is accelerated, and the operation is simple; and meanwhile, the HPLC purity of the obtained hydroxychloroquine refined product is not less than 96.50%, the maximum single impurity content is less than 0.10%, the yield can reach 85%, the HPLC purity of hydroxychloroquine sulfate is not less than 98.00%, the maximum single impurity content is less than 0.10%, and the yield can reach 90%.
Owner:JIANGXI GUOYAO PHARMA LLC +1
Preparation method of chloroquine phosphate
The invention discloses a preparation method of chloroquine phosphate, namely 7-chloro-4-(4-diethylamino-1-methylbutylamino)quinoline diphosphate. The preparation method comprises the following steps: (1) carrying out a condensation reaction on 4,7-dichloroquinoline and 2-amino-5-diethylaminopentane, and carrying out alkalization extraction, concentration and crystallization to obtain chloroquine; and (2) salifying the chloroquine obtained in the step (1) and phosphoric acid to obtain chloroquine phosphate. The method avoids the use of phenol, is simple in reaction, and is beneficial to industrial production; and the chloroquine is crystallized to improve the purity and then salified with the phosphoric acid, so product appearance is good, the purity of the produced chloroquine phosphate is greater than or equal to 99.5%, and a single impurity is less than 0.1% (according to an HPLC area normalization method).
Owner:CHONGQING KANGLE PHARMA
Synthesis method of 4,7-dichloroquinoline
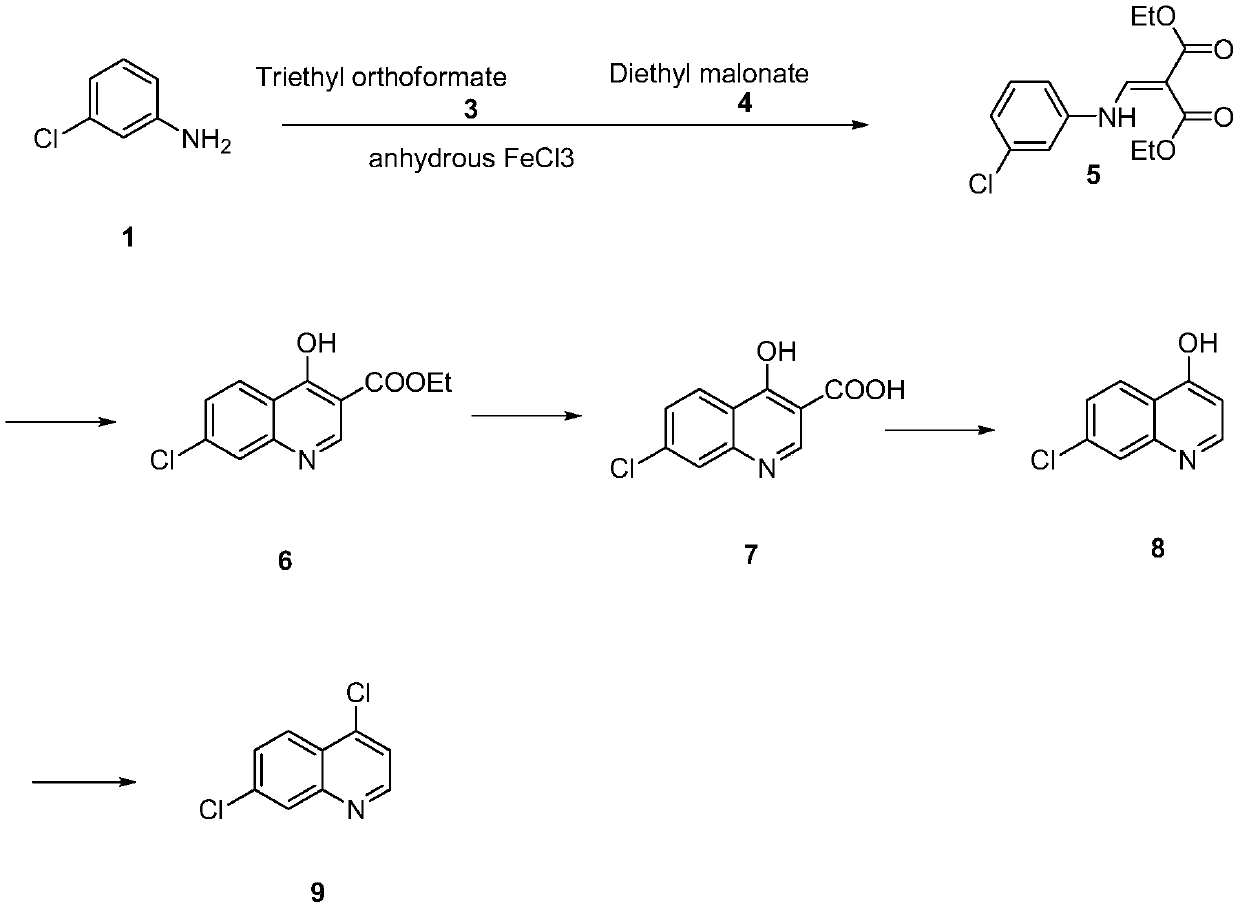
The invention discloses a synthesis method of 4,7-dichloroquinoline. The synthesis method is characterized by comprising the following steps: synthesizing 7-chloro-4-hydroxylquinoline-3-carboxylic acid by using a one-pot method, and carrying out decarboxylation and chlorination on the 7-chloro-4-hydroxylquinoline-3-carboxylic acid to obtain 4,7-dichloroquinoline. The step of synthesizing the 7-chloro-4-hydroxylquinoline-3-carboxylic acid by the one-pot method comprises the following sub-steps: with m-chloroaniline, triethyl orthoformate or trimethyl orthoformate and diethyl malonate as raw materials, carrying out condensation under the catalysis of anhydrous ferric trichloride to obtain diethyl 2-[[(3-chlorophenyl)amino]methylene]malonate, directly adding a condensation reaction solution into an organic solvent, carrying out heating cyclization to obtain 7-chloro-4-hydroxylquinoline-3-carboxylic acid ethyl ester, and after the cyclization reaction is completed, adding sodium hydroxidefor hydrolysis to obtain 7-chloro-4-hydroxylquinoline-3-carboxylic acid. Although the whole process comprises five reactions, intermediate products are good enough in purity and can be directly synthesized into a target product without purification, so operation is easy and convenient and industrialization is facilitated; and raw materials are easily available, and pollution is small.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
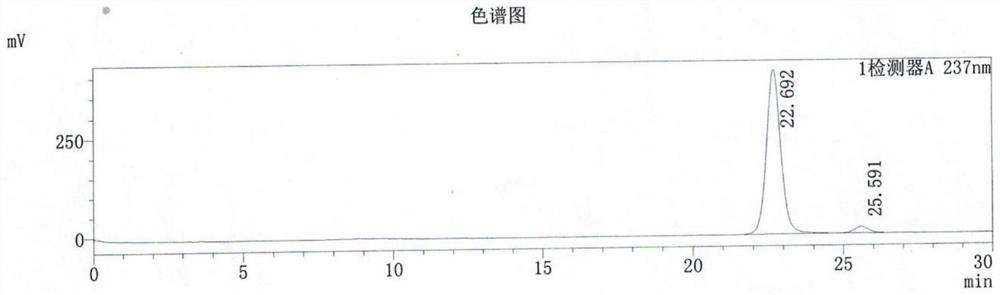
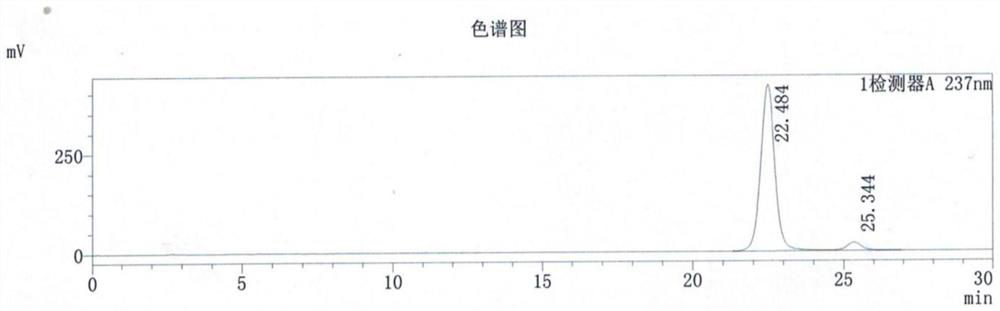
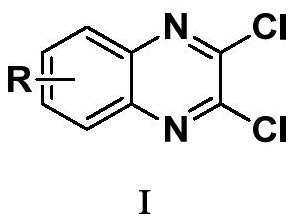
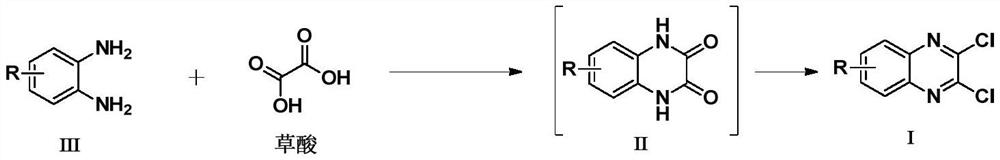
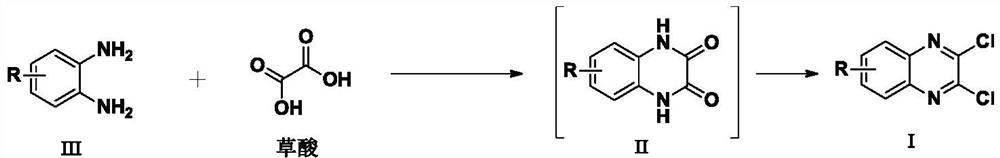
Synthesis method of 3-ethyl carboxylate-4-chloro imidazole [1,5-a] quinoxaline
The invention relates to a synthesis method of 3-ethyl carboxylate-4-chloro imidazole [1,5-a] quinoxaline, which is mainly used for solving the technical problems of the existing scarce synthetic route, expensive reaction raw materials, difficulty in amplification, narrow application range and the like. The synthesis method comprises the following steps: heating o-phenylenediamine 1 which is a cheap and available chemical raw material and diethyl oxalate for reacting in a solvent to obtain a compound 2 which is 2,3-dihydroxyl quinoxaline; heating the compound 2 and a chloride reagent for reacting to obtain a compound 3 which is 2,3-dichloro-quinoxaline; and reacting the compound 3 with ethyl isocyanoacetate in a solvent in the presence of an alkalization reagent to obtain the final product, namely 3-ethyl carboxylate-4-chloro imidazole [1,5-a] quinoxaline. By adopting the synthetic route provided by the invention, a large quantity of the 3-ethyl carboxylate-4-chloro imidazole [1,5-a] quinoxaline can be quickly and conveniently prepared.
Owner:上海药明康德新药开发有限公司 +1
Preparation method of 4, 7-dichloroquinoline
ActiveCN112194621AGuaranteed qualityHigh yieldOrganic chemistryBenzoic acidDiethyl ethoxymethylenemalonate
The invention relates to a preparation method of 4, 7-dichloroquinoline in the technical field of medicines, and the method comprises the following steps of: carrying out condensation reaction on 2-amino-6-chlorobenzoic acid (I) serving as an initial raw material and diethyl ethoxymethylenemalonate (II) in an organic solvent to obtain (III), and carrying out cyclizing, hydrolyzing and acidifying on (III) to obtain hydroxyquinoline dicarboxylic acid (V). performing high-temperature decarboxylation on V to obtain hydroxyquinoline (VI), and chlorinating VI with phosphorus oxychloride to obtain 4,7-dichloroquinoline. The 4, 7-dichloroquinoline prepared by the method disclosed by the invention does not contain isomer 4, 5-dichloroquinoline, and the temperature of the condensation reaction is low under the catalysis of the catalyst, so that the reaction yield is improved, the generation of impurities is avoided, the energy consumption is reduced, and the economic benefit of the product is improved.
Owner:JIANGSU TIANHE PHARMA CO LTD
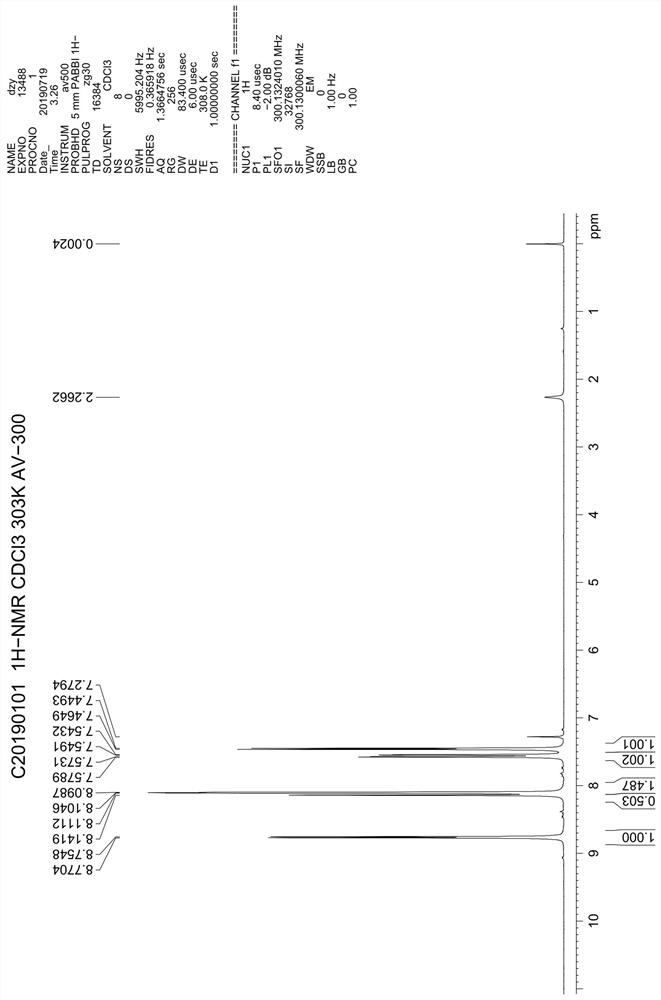
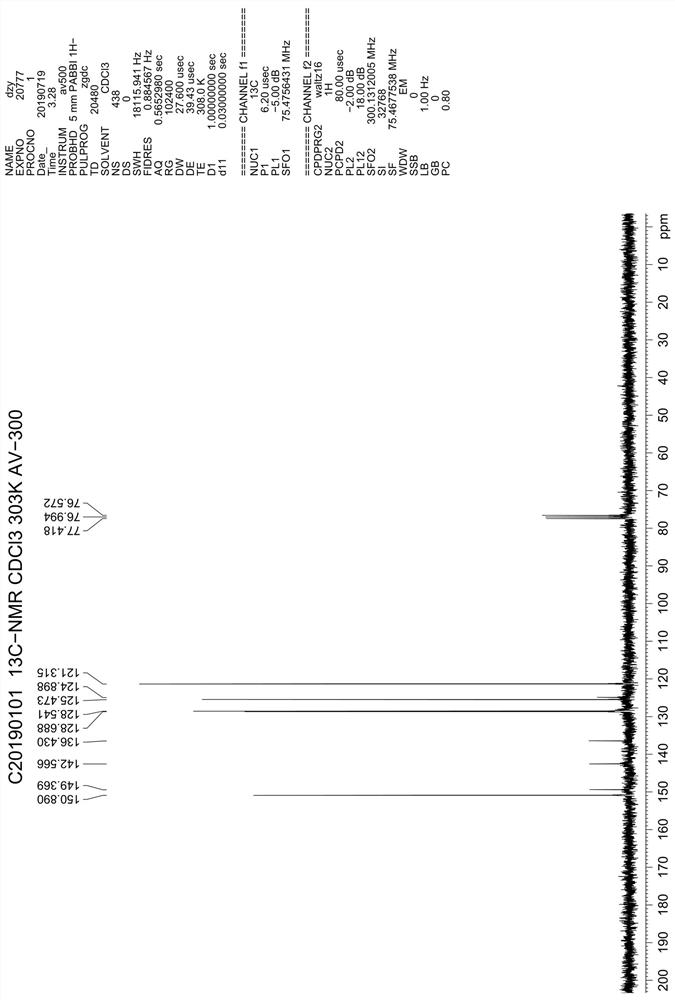
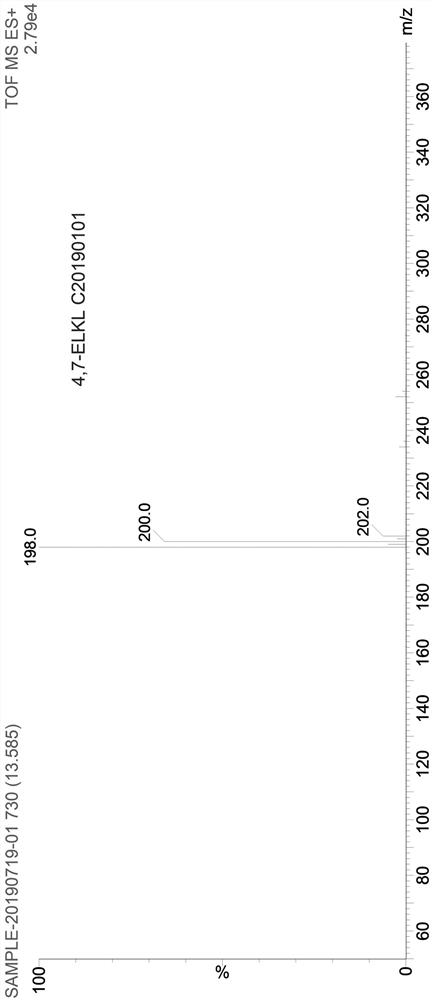
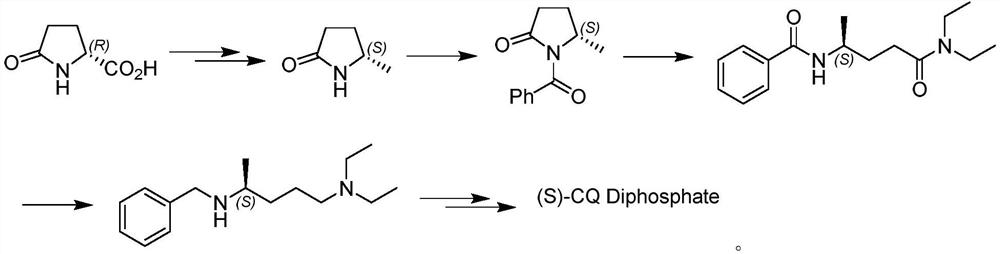
Asymmetric synthesis method of (S)-chloroquine phosphate
PendingCN112266356AFew reaction stepsHigh selectivityOrganic chemistry methodsO-Phosphoric AcidSide chain
The invention provides a novel asymmetric synthesis method of (S) chloroquine. According to the invention, 5-(N-diethylamino)-2-pentanone and (S)-alpha-methyl benzylamine are adopted as raw materials,and in the presence of Lewis acid, an asymmetric reductive amination reaction is carried out to obtain (S,S)-5-(N'-diethylamino)-N-((1-phenyl)ethyl)-2-pentylamine, catalytic hydrogenation is performed to remove benzyl to obtain a side chain (S)-5-(N'-diethylamino)-2-amyl amine for synthesizing chloroquine, the (S)-5-(N'-diethylamino)-2-amyl amine is condensed with 4,7-dichloroquinoline to obtain(S)-chloroquine, salifying is performed with phosphoric acid, and recrystallizing is performed to obtain (S)-chloroquine phosphate with ee value of more than 99%, wherein the the total yield of the four-step reaction exceeds 70%; and the preparation method is stable, reliable, economical, efficient and easy to industrialize.
Owner:江苏百奥信康医药科技有限公司
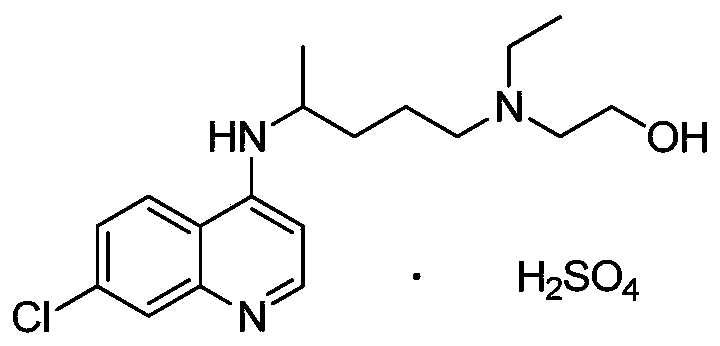
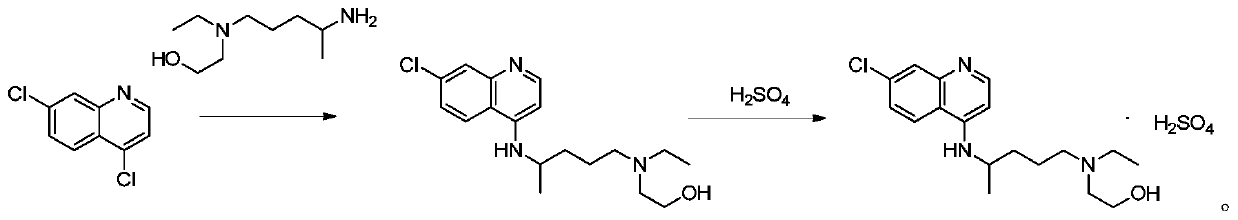
Preparation method of hydroxychloroquine sulfate
PendingCN111423373ASimple processGood reproducibilityOrganic chemistryHydroxychloroquineReaction temperature
The invention relates to a preparation method of hydroxychloroquine sulfate, and belongs to the technical field of medicine synthesis. According to the preparation method of hydroxychloroquine sulfate, 7-chloro-4-fluoroquinoline and 5-(N-ethyl-N-2-hydroxyethyl amine)-2-pentylamine are subjected to a reaction to prepare hydroxychloroquine, and then hydroxychloroquine and sulfuric acid are salifiedto prepare hydroxychloroquine sulfate. The 7-chloro-4-fluoroquinoline is prepared from 4,7-dichloroquinoline through a halogen exchange reaction. The preparation method of hydroxychloroquine sulfate has the advantages of low reaction temperature, short reaction time, fewer byproducts, simple technique and favorable reproducibility, and is beneficial to industrial production.
Owner:REYOUNG PHARMA
Weeding composition containing dichloroquinoline oxadiazon
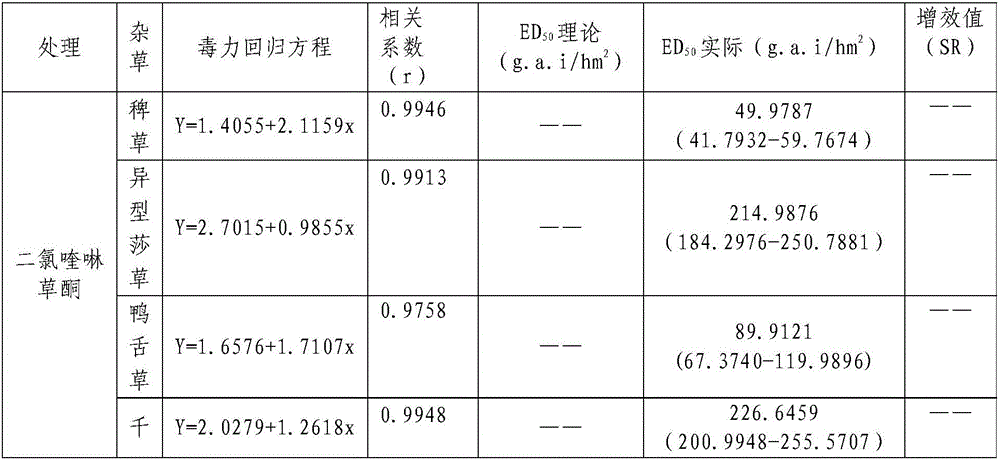
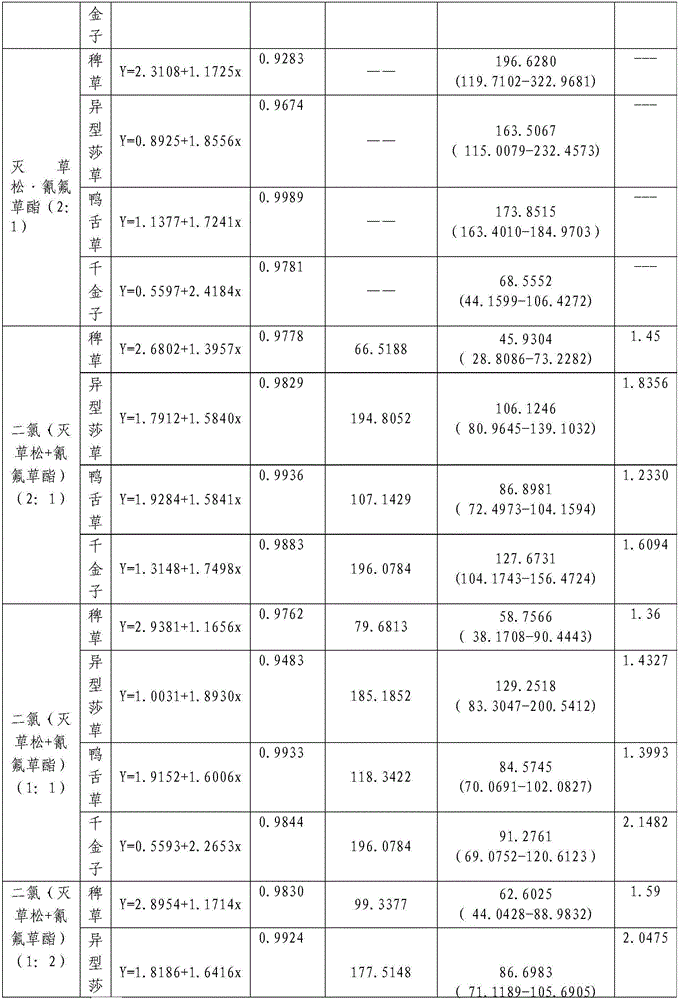
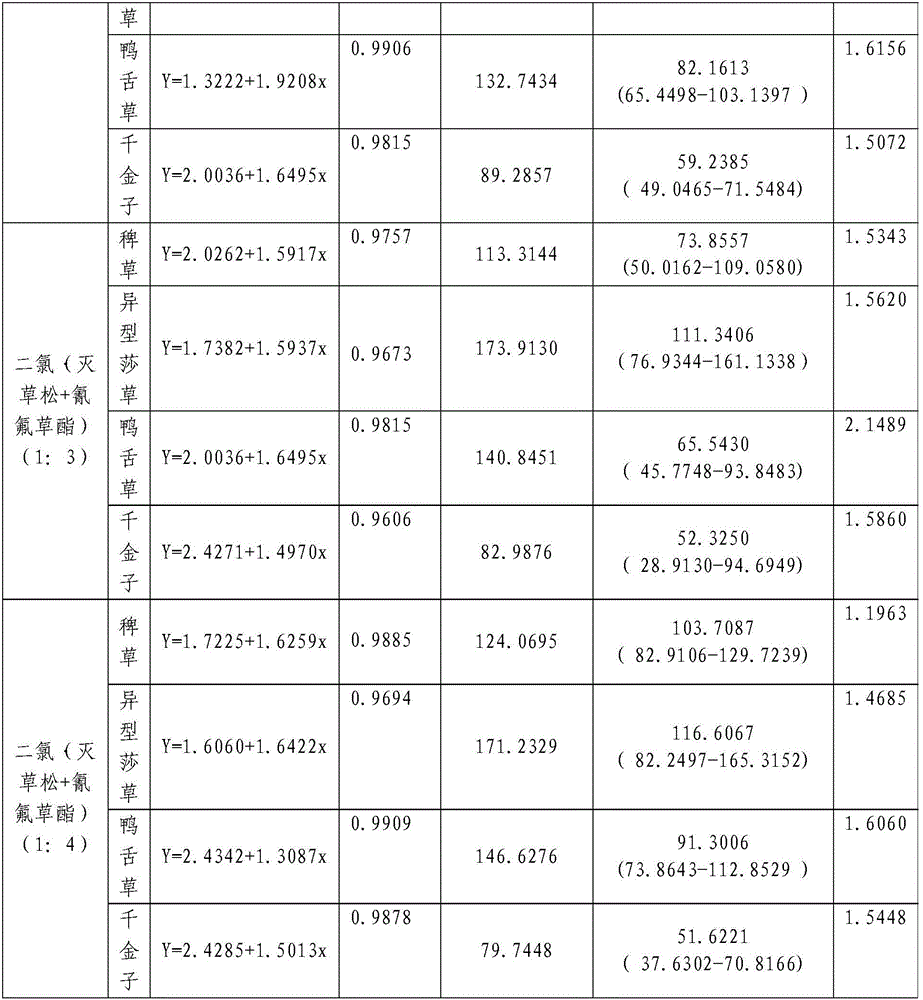
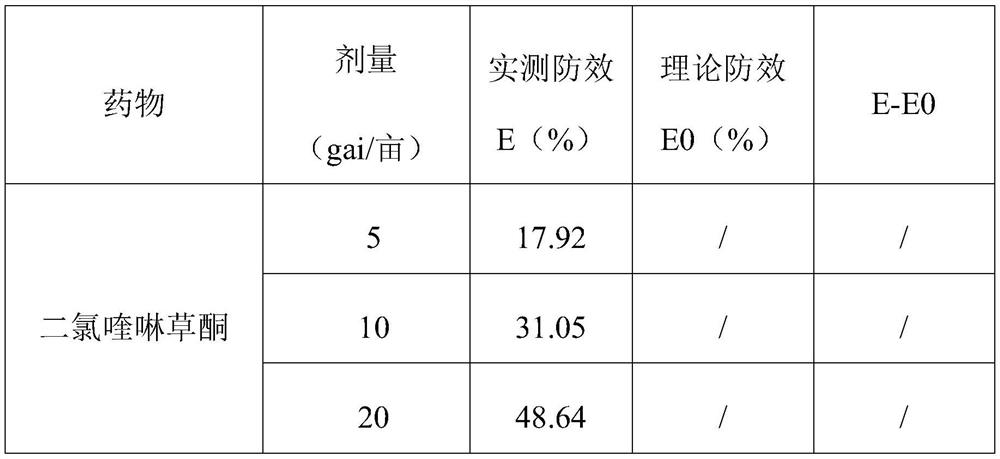
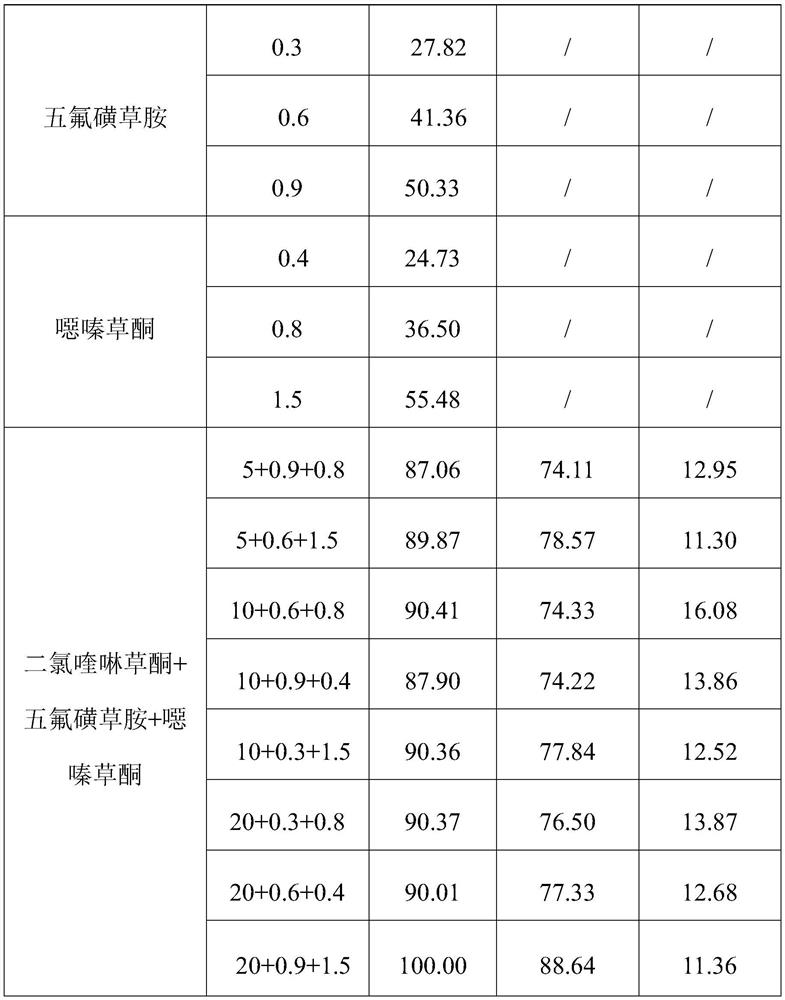
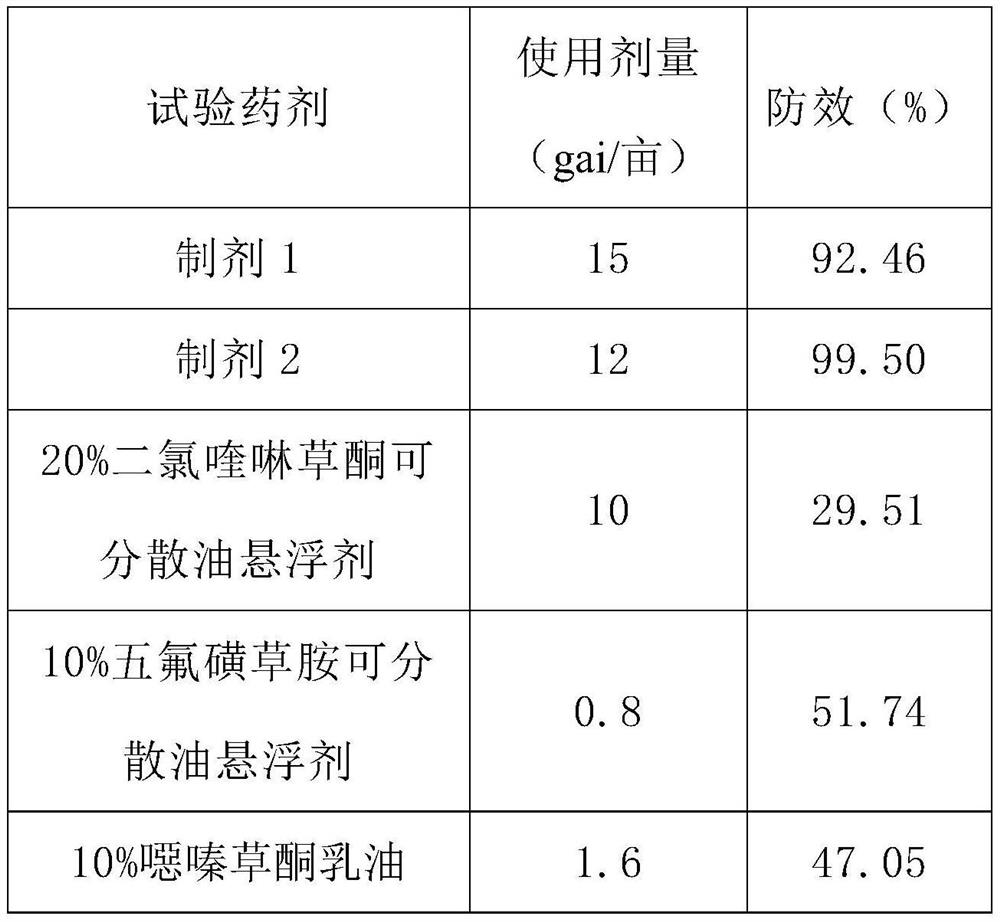
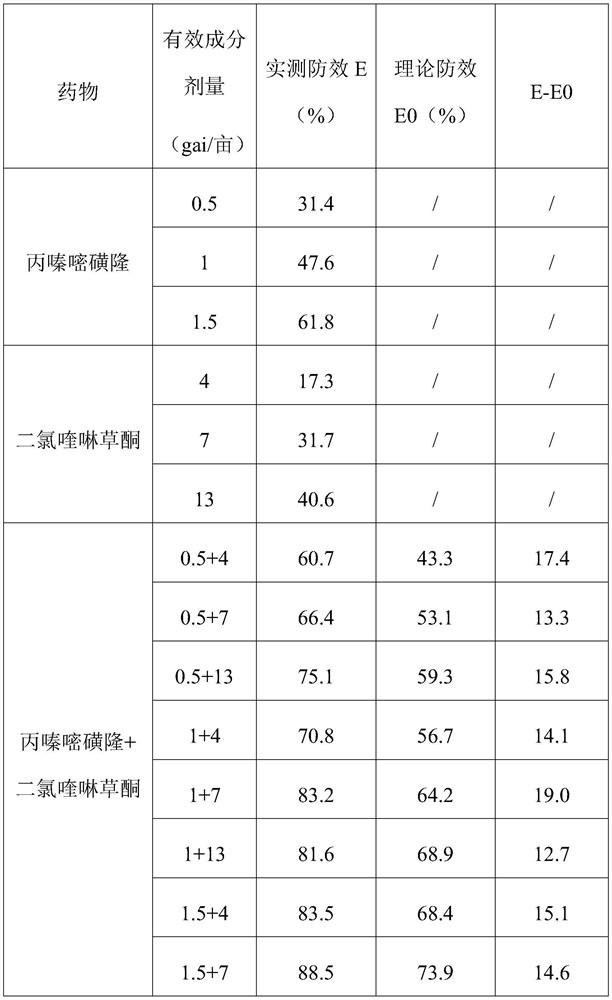
InactiveCN106719718AGood control effectGood weeding effectBiocideDead animal preservationTreatment effectDrug resistance
The invention discloses a weeding composition containing dichloroquinoline oxadiazon. Active components of the weeding composition containing dichloroquinoline oxadiazon comprise dichloroquinoline oxadiazon, bentazone and cyhalofop-butyl. According to the weeding composition disclosed by the invention, due to different weeding mechanisms of dichloroquinoline oxadiazon, bentazone and cyhalofop-butyl, the complex synergism effect of dichloroquinoline oxadiazon, bentazone and cyhalofop-butyl is obvious, and the herbicide controlling spectrum is wide; the weeding composition containing dichloroquinoline oxadiazon is relatively good in prevention and treatment effect on broadleaf weeds, cyperus weeds and gramineous weeds; the drug use amount is reduced, so that the cost is reduced, and the weeding composition is safer; meanwhile, the drug resistance generated by the use of a single dosage can be relieved.
Owner:PLANT PROTECTION & QUALITY & SAFETY OF AGRI PRODS INST ANHUI ACAD OF AGRI SCI
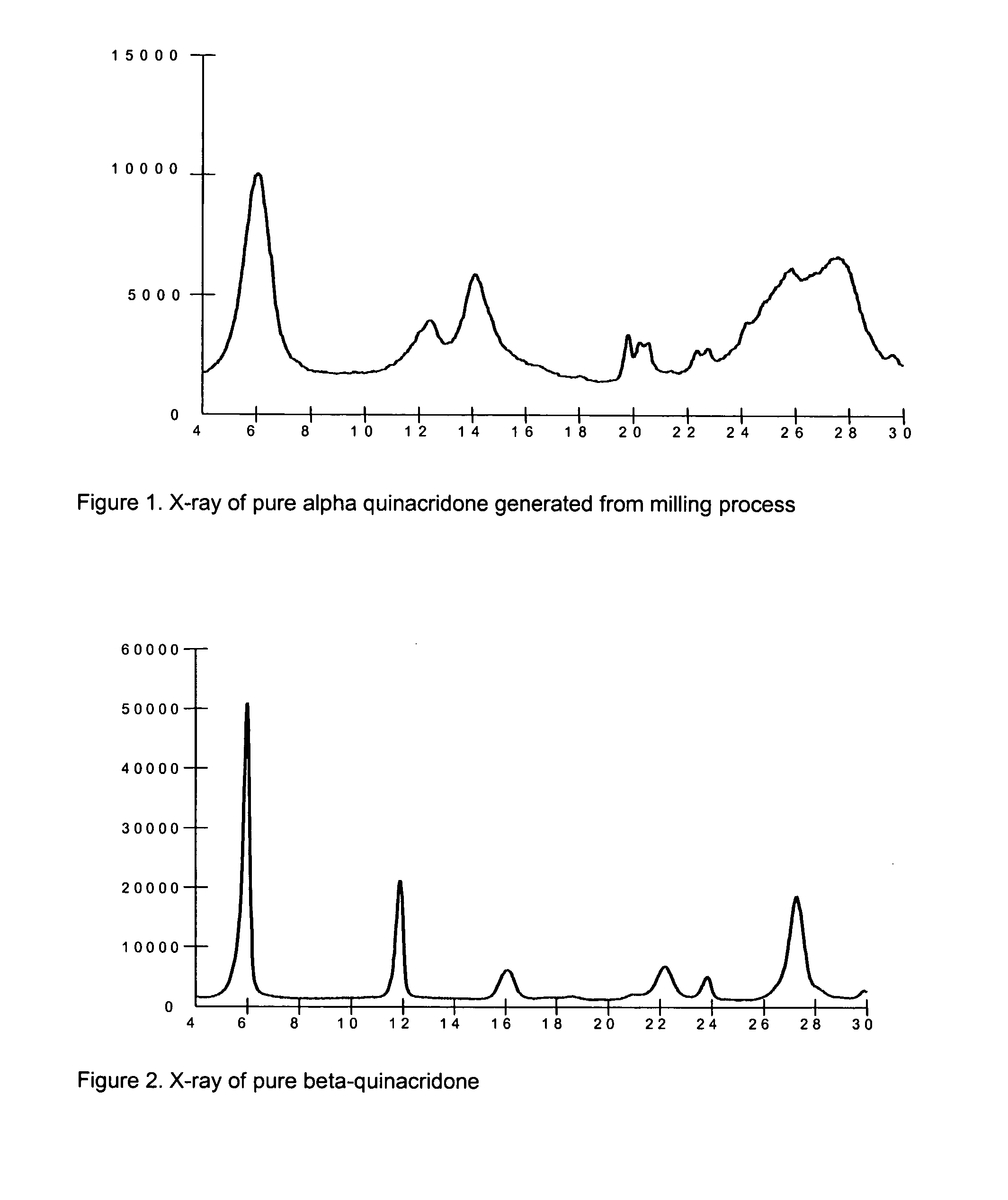
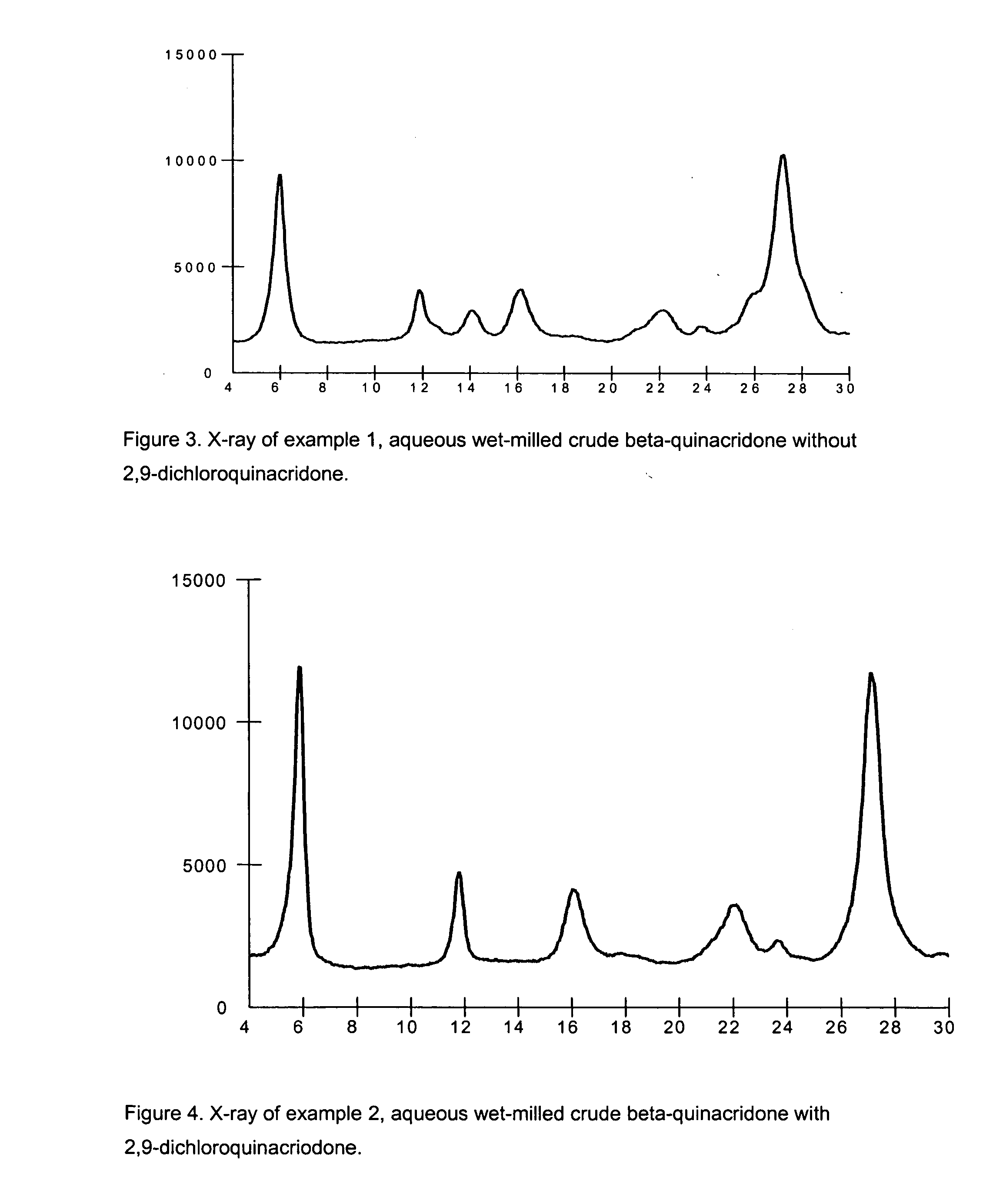
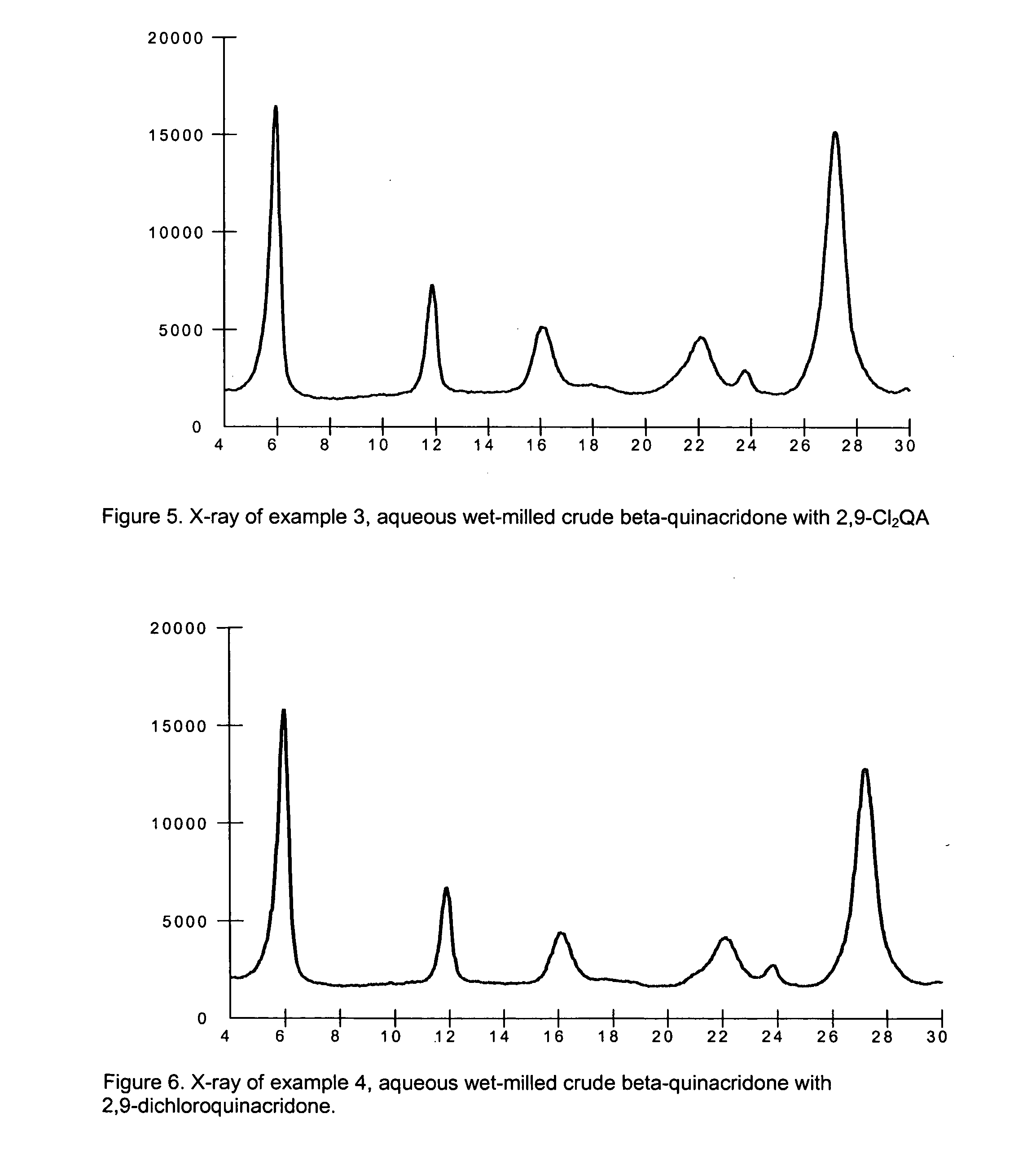
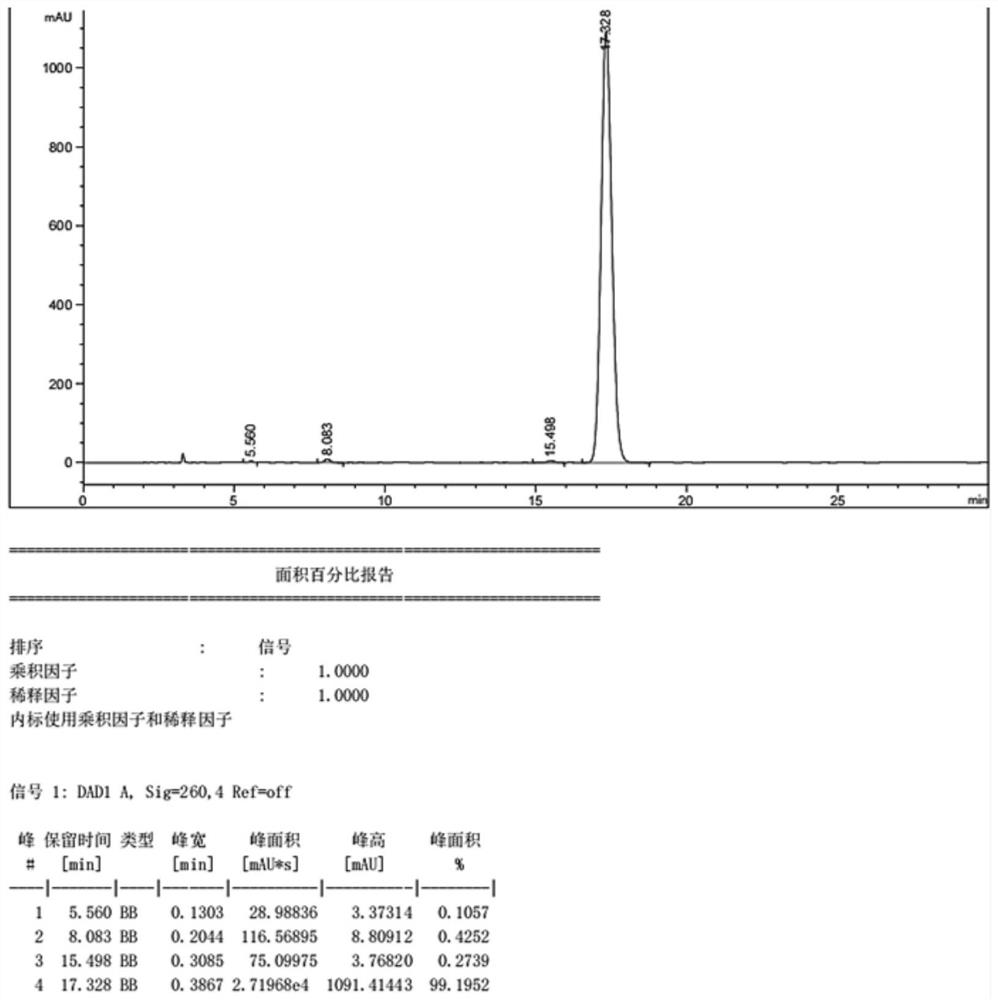
2,9-Dichloro-quinacridone as alpha-quinacridone crystal phase inhibitor
ActiveUS20050183635A1Inhibition formationPigment propertyOrganic chemistryInksQuinacridoneSize reduction
The invention is directed to a method or use of 2,9-dichloro-quinacridone as a crystal phase inhibitor during the beta-quinacridone or gamma-quinacridone crude pigment particle size reduction processes. 2,9-dichloroquinacridone is added to the milling composition of the crude gamma or crude beta quinacridone
Owner:CIBA SPECIALTY CHEM CORP
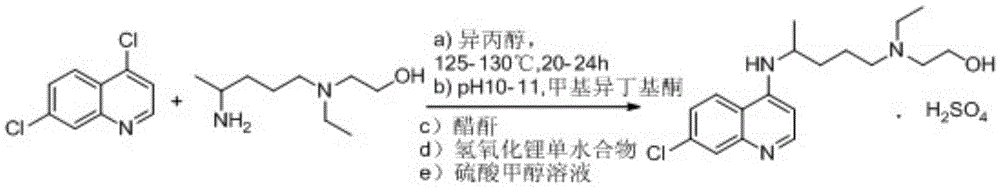
Preparation method of hydroxychloroquine sulfate
PendingCN114478377ASimple processing methodEasy to operateOrganic chemistryHydroxychloroquineSide chain
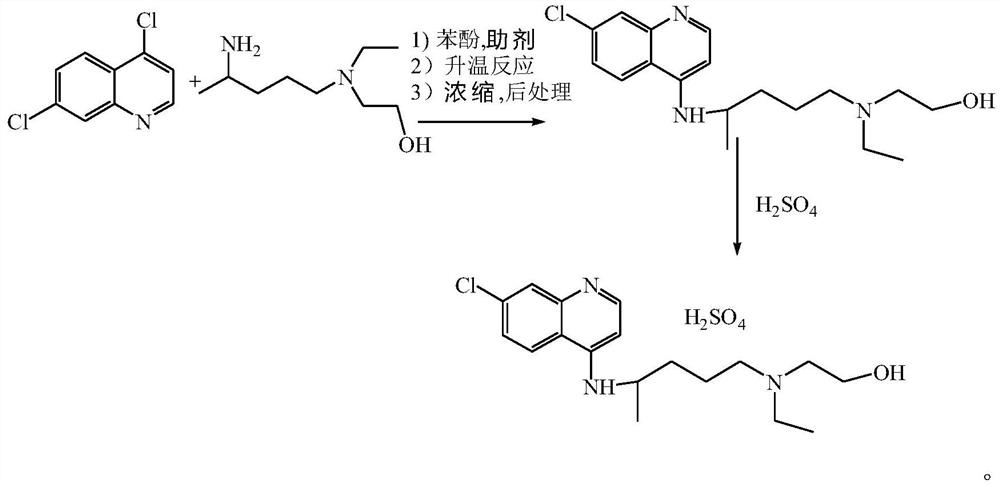
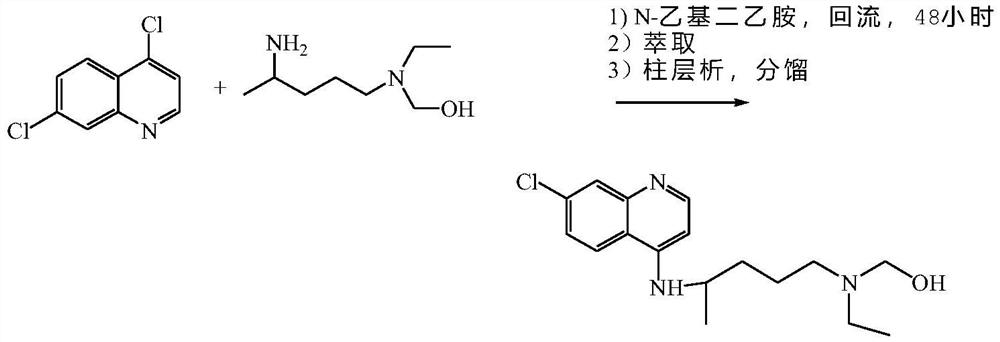
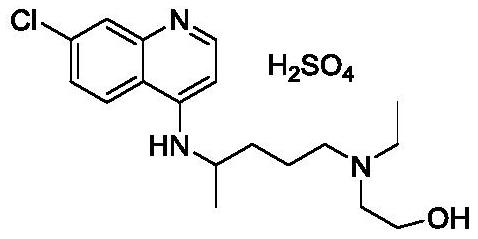
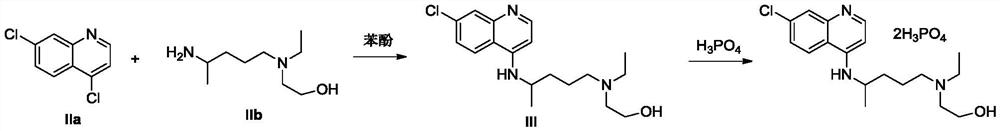
The invention discloses a preparation method of hydroxychloroquine sulfate, and belongs to the technical field of medicine synthesis. The method comprises the following steps: (1) mixing 4, 7-dichloroquinoline, an auxiliary agent and phenol, heating, adding a side chain, and reacting to obtain a hydroxychloroquine crude product; (2) purifying the hydroxychloroquine crude product obtained in the step (1) to obtain hydroxychloroquine; and (3) dissolving the hydroxychloroquine obtained in the step (2), and adding sulfuric acid to obtain hydroxychloroquine sulfate. The preparation method can shorten the reaction time, reduce the reaction temperature, reduce the generation of impurities, greatly reduce the production cost and reduce the environmental pollution, and is simple to operate and suitable for industrial production.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Preparation method of hydroxychloroquine
PendingCN112745263AHigh yieldHigh purityOrganic chemistryBulk chemical productionHydroxychloroquineQuinoline
The invention relates to a preparation method of hydroxychloroquine, which comprises the following steps: protecting hydroxyl of 5-(N-ethyl-N-ethoxyl)-2-aminopentane through a silanization reagent, removing amino protons from tetrahydrofuran or toluene by using a bis(trimethylsilyl lithium amide) solution to form amino anions, and carrying out a substitution reaction with 4.7 dichloroquinoline to generate hydroxychloroquine. The hydroxychloroquine and sulfuric acid are salified in an alcoholic solution to generate hydroxychloroquine sulfate, and the hydroxychloroquine sulfate preparation method provided by the invention has the characteristics of low toxicity, low pollution, high purity, low reaction temperature, short reaction time, high yield and the like, and is suitable for industrialization.
Owner:NANJING GRITPHARMA CO LTD
New synthesis method of high-content quizalofop-ethyl intermediate
InactiveCN102775359AReduce oxidation lossReduce difficultyOrganic chemistryOrganosolvHydroquinone Compound
The invention discloses a new synthesis process of a quizalofop-ethyl pesticide intermediate high-purity 2-(4-hydroxyphenoxy)-6-chloroquinoxalin. The synthesis process comprises the following steps of: adding 2, 6-dichloroquinoxaline and hydroquinone into a mixed solvent; heating the mixed solvent, and dripping liquid alkali; carrying out thermal insulation reaction after dripping, separating out an organic solvent after reaction; and cooling, discharging and washing to obtain a high-purity target. With the adoption of the process, the excess ratio of hydroquinone and the sewage production difficulty are greatly lowered. The new synthesis process is a green process.
Owner:ANHUI FENGLE AGROCHEM
A kind of herbicidal composition containing quinclomid and its application
Owner:安徽尚禾沃达生物科技有限公司
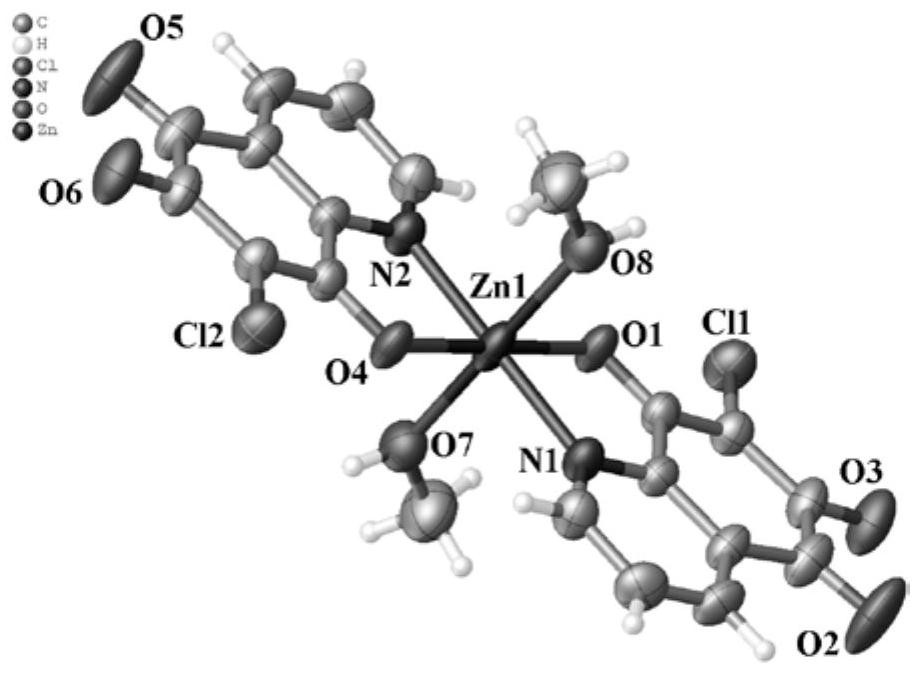
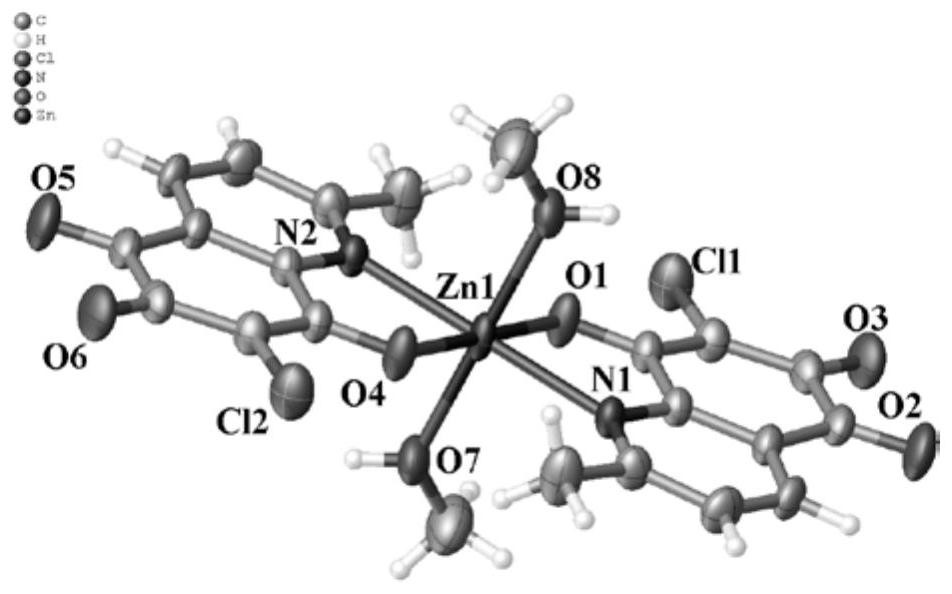
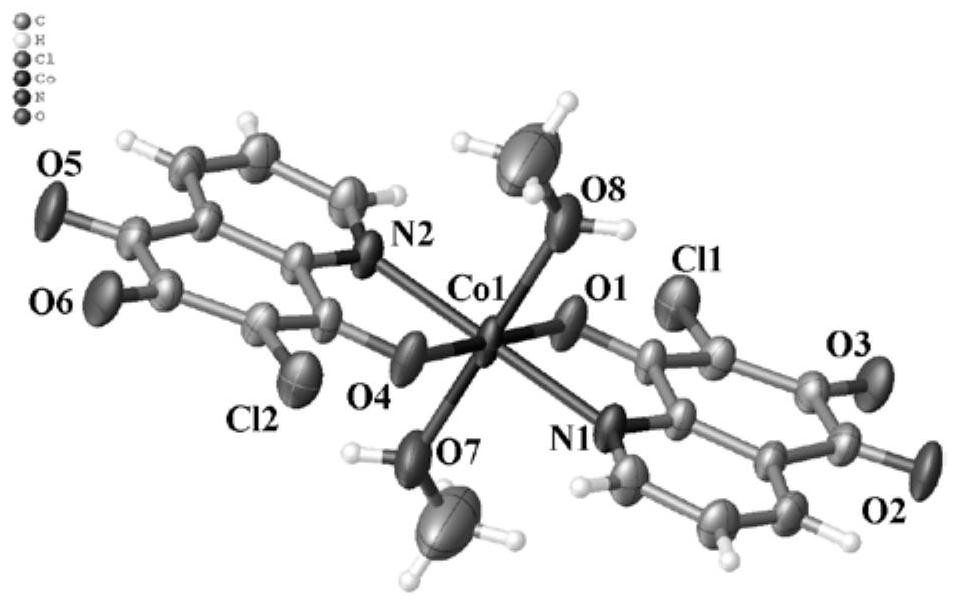
6,7-dichloroquinoline-5,8-dione derivative transition metal complex and its synthesis method and application
ActiveCN110423242BGood antitumor activityLow toxicityCopper organic compoundsNickel organic compoundsQuinolineTumor cells
The invention discloses a class of 6,7-dichloroquinoline-5,8-dione derivative transition metal complexes and a synthesis method and application thereof. The complex of the present invention has the structure shown in the following formula (I), and its synthesis method is to put metal nitrate and the compound shown in the following formula (II) in a mixed solvent, and carry out coordination under heating or without heating reaction, the reactant is cooled, allowed to stand, volatilized, and crystals are precipitated to obtain the target compound; wherein, the mixed solvent is a composition of methanol or ethanol and dichloromethane; the complex of the present invention is effective for certain tumor cells The strain has significant inhibitory activity and has low toxicity to normal human liver cells. The compounds shown in the formula (I) and the formula (II) are respectively as follows: wherein, M represents a divalent metal element selected from the fourth period of the periodic table; R represents H or CH 3 , X means H 2 O, CH 3 OH or CH 3 CH 2 Oh.
Owner:GUILIN NORMAL COLLEGE
A kind of mixed herbicide containing promasulfuron-methyl and quinclozone and its application
ActiveCN108935504BDelay drug resistanceExpand the spectrum of weed controlBiocideAnimal repellantsBiotechnologyPromethazine
The invention provides a mixed herbicide containing propyrisulfuron and dichloroquinolone and an application thereof. The mixed herbicide contains active components of the propyrisulfuron and the dichloroquinolone; the weight ratio of the propyrisulfuron to the dichloroquinolone is 1-10:1-40. The mixed herbicide provided by the invention can be used for controlling broadleaf weeds, gramineous weeds and nutgrass flatsedge.
Owner:ANHUI YOUNGSUN PESTICIDES
A kind of preparation method of 4,7-dichloroquinoline
The invention relates to a preparation method of 4,7-dichloroquinoline in the technical field of medicine, which uses 2-amino-6-chlorobenzoic acid (I) as a starting material, and ethoxymethine in an organic solvent Diethyl malonate (II) undergoes condensation reaction to obtain (Ⅲ), III undergoes cyclization, hydrolysis, and acidification to obtain hydroxyquinoline dicarboxylic acid (V), V undergoes high-temperature decarboxylation to obtain hydroxyquinoline (VI), and VI undergoes Chlorination of phosphorus oxychloride gives 4,7-dichloroquinoline. The 4,7-dichloroquinoline prepared by the method of the present invention does not have the isomer 4,5-dichloroquinoline, and the temperature of the condensation reaction under the catalysis of the catalyst is low, which improves the yield of the reaction and avoids the generation of impurities , and reduce energy consumption, improve the economic benefits of the product.
Owner:JIANGSU TIANHE PHARMA CO LTD
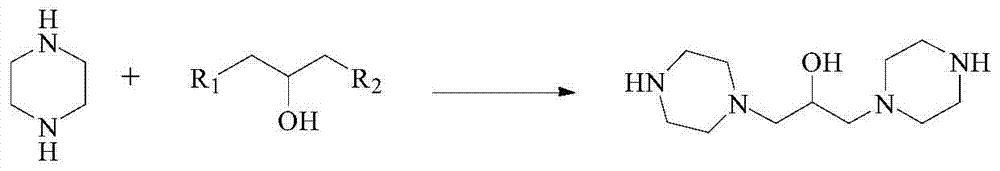
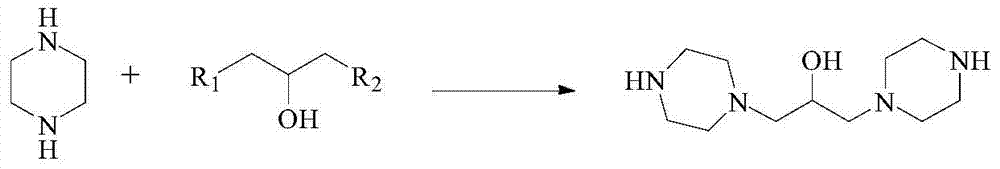
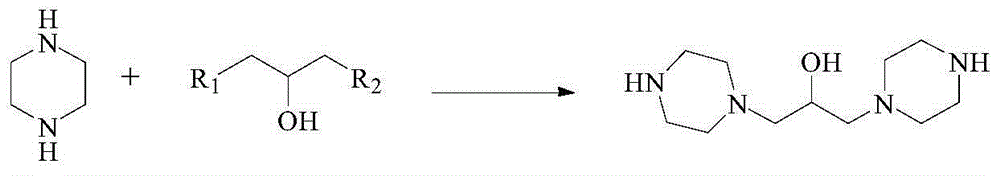
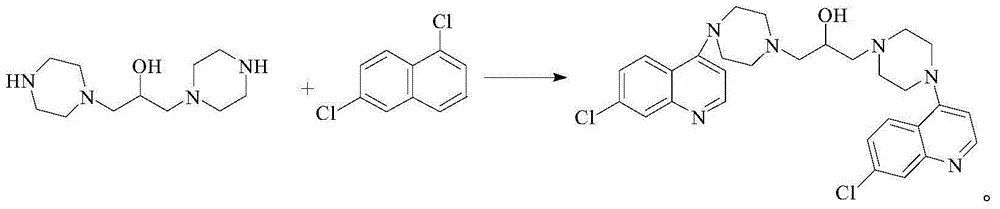
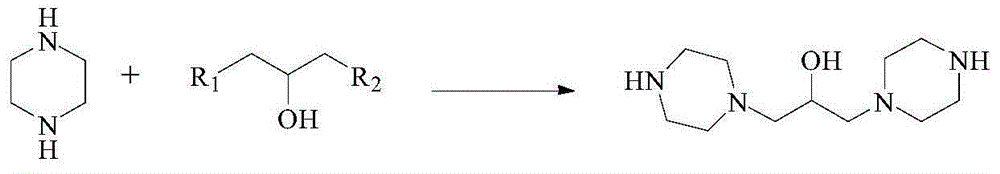
Preparation method of hydroxypiperaquine and phosphate thereof
The invention provides a preparation method of hydroxypiperaquine and phosphate thereof. The preparation method of hydroxypiperaquine comprises the following steps: by using piperazine, 1,3-dihalopropanol and 4,7-dichloroquinoline as raw materials, reacting the piperazine and 1,3-dihalopropanol by using an acid-binding agent as a catalyst to obtain 1,3-dipiperazinylpropanol, carrying out condensation reaction on the 1,3-dipiperazinylpropanol and 4,7-dichloroquinoline under the catalytic action of an alkali to obtain the hydroxypiperaquine. The obtained hydroxypiperaquine reacts with phosphoric acid to obtain the hydroxypiperaquine phosphate. The method has the advantages of mild reaction conditions, simple preparation technique and low cost, and is suitable for industrial production. The total reaction yield of the hydroxypiperaquine is up to 90-91.5%, and the total reaction yield of the hydroxypiperaquine phosphate is up to 82.7-87.5%.
Owner:上海博速医药科技有限公司
Synthesis method of quizalofop-p-ethyl
PendingCN114478406AAvoid racemizationNot easy to racemizeOrganic chemistryPropanoic acidPtru catalyst
The invention relates to a preparation method of quizalofop-p-ethyl, which comprises the following steps: reacting 2, 6-dichloroquinoxaline (II) with R (+) 2-(4-hydroxyphenoxy) ethyl propionate (III) in the presence of an organic solvent, an acid-binding agent and a catalyst until the 2, 6-dichloroquinoxaline is less than or equal to 0.5%, and ending the reaction to obtain the quizalofop-p-ethyl (I) with high optical content, the catalyst is selected from one or a mixture of more of tetrabutylammonium bromide, benzyltriethylammonium chloride and N, N-dimethylformamide. According to the method disclosed by the invention, the specific catalyst is added, and the highest reaction temperature is adjusted to 85 DEG C or below, so that the reaction time is greatly shortened, and the racemization problem caused by long-time reaction of quizalofop-p-ethyl in a high-temperature and strong-alkali environment is avoided; meanwhile, water is separated through reduced pressure reflux while reacting, water generated in the reaction is removed in time, product hydrolysis is avoided, the reaction rate and yield are increased, and the method has high industrialization value.
Owner:HAIZHENG CHEM NANTONG CO LTD
Preparation method of hydroxypiperaquine and phosphate thereof
ActiveCN105111142AEasy to prepareEasy to operateOrganic chemistryPhosphoric acidHydroxypiperaquine phosphate
The invention provides a preparation method of hydroxypiperaquine and phosphate thereof. The preparation method of hydroxypiperaquine comprises the following steps: by using piperazine, 1,3-dihalopropanol and 4,7-dichloroquinoline as raw materials, reacting the piperazine and 1,3-dihalopropanol by using an acid-binding agent as a catalyst to obtain 1,3-dipiperazinylpropanol, carrying out condensation reaction on the 1,3-dipiperazinylpropanol and 4,7-dichloroquinoline under the catalytic action of an alkali to obtain the hydroxypiperaquine. The obtained hydroxypiperaquine reacts with phosphoric acid to obtain the hydroxypiperaquine phosphate. The method has the advantages of mild reaction conditions, simple preparation technique and low cost, and is suitable for industrial production. The total reaction yield of the hydroxypiperaquine is up to 90-91.5%, and the total reaction yield of the hydroxypiperaquine phosphate is up to 82.7-87.5%.
Owner:上海博速医药科技有限公司
Production method and use for dichloro quinolinic acid artificial hapten, artificial antigen and specific antibody
InactiveCN1240685CEasy to handleFast and accurate analysis and detectionImmunoglobulinsTesting foodAntiendomysial antibodiesSpecific antibody
The invention discloses a process for preparing Quinclorac artificial semiantigen, artificial antigen, specific antibody and use thereof, wherein the preparation comprises, subjecting the dichloroquine (3,7-dichlorine-8-quinoline carboxylic acid) to sulfoxide chlorinated acylation, reacting with reanal and aminocaproic acid, thus obtaining semiantigen 4-(3,7-dichlorine-8-quinolineformyl) reanal or 6-(3,7-dichlorine-8-quinolineformyl) aminocaproic acid (QB or QC).
Owner:ZHEJIANG UNIV
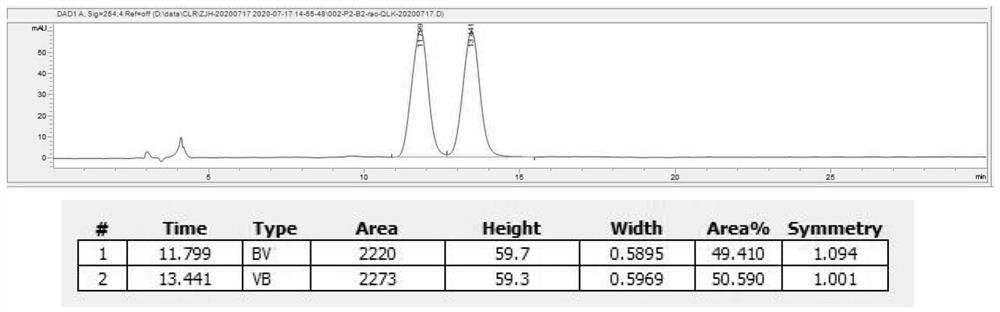
Asymmetric synthesis method of optically pure (R)/(S)-hydroxychloroquine side chain
The invention provides a method for synthesizing optically pure (R) / (S)-hydroxychloroquine, which comprises the following steps: resolving (+ / -)-2-[(4-aminoamyl)ethylamino]ethanol by using a resolving reagent, recrystallizing and purifying the compound to obtain optically pure (R) / (S)-2-[(4-aminoamyl)ethylamino]ethanol; and reacting the obtained single-configuration hydroxychloroquine side chain with 4,7-dichloroquinoline to obtain the hydroxychloroquine sulfate of single configuration. The method has the advantages of simple route, easily available raw materials, high yield, good stereoselectivity (ee% is greater than 99%), simple operation, greenness and environmental protection, and is suitable for large-scale production.
Owner:SHENZHEN CATALYS SCI & TECH CO LTD
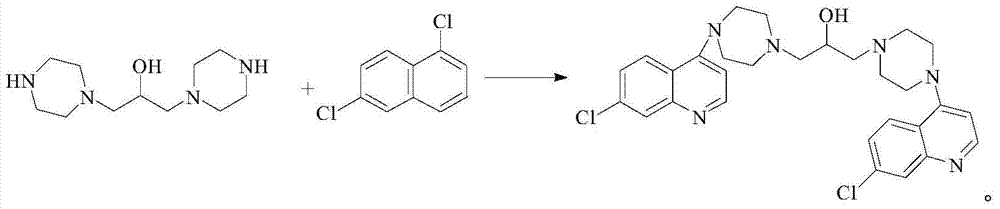
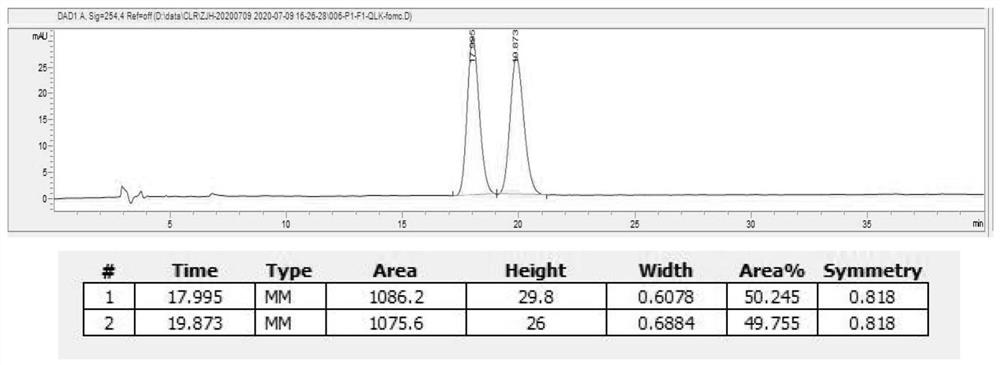
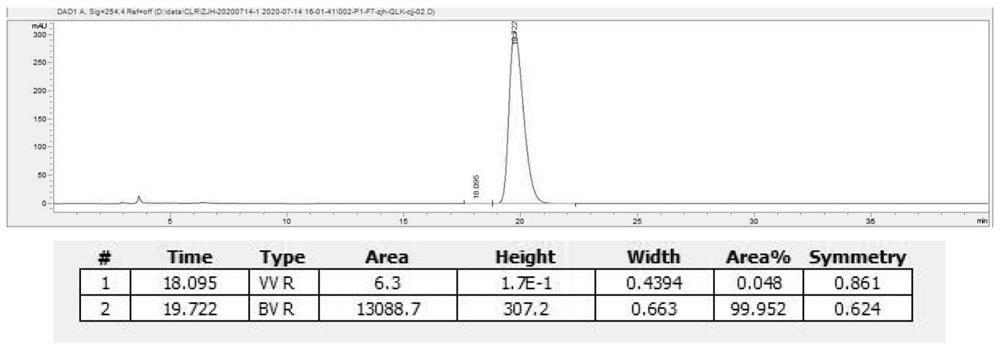
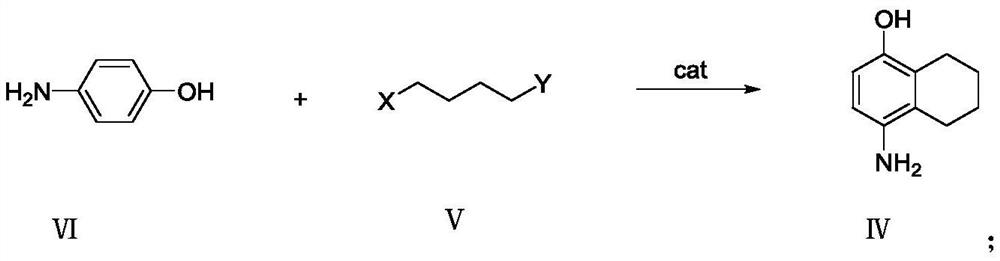
Preparation method of naphthoquine phosphate
The invention discloses a preparation method of naphthoquine phosphate formula I. The preparation method is characterized by comprising the following steps: reacting p-aminophenol formula VI with 1, 4-disubstituted butane formula V to obtain 4-amino-5, 6, 7, 8-tetrahydro-1-naphthol formula IV, and reacting the formula IV with 4, 7-dichloroquinoline formula VII to obtain 4-(7-chloro-4-quinoline amino)-5, 6, 7, 8-tetrahydro-1-naphthol formula III, reacting the formula III with formaldehyde and tert-butylamine to obtain naphthoquine basic formula II, and salifying the naphthoquine basic formula II with phosphoric acid to obtain naphthoquine phosphate formula I; the process route has the advantages of simple and accessible raw materials, high yield and low pollution and is suitable for industrial production.
Owner:CHONGQING KANGLE PHARMA
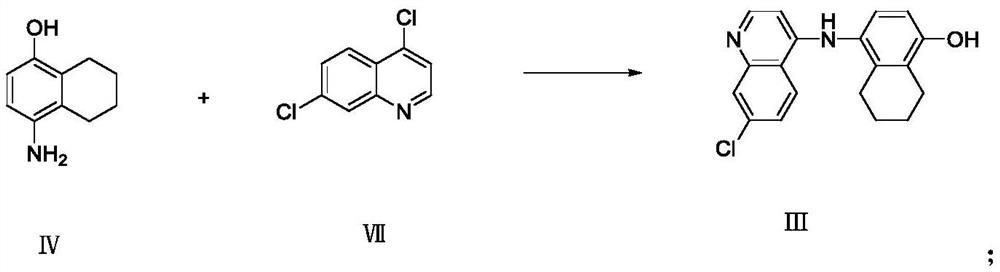
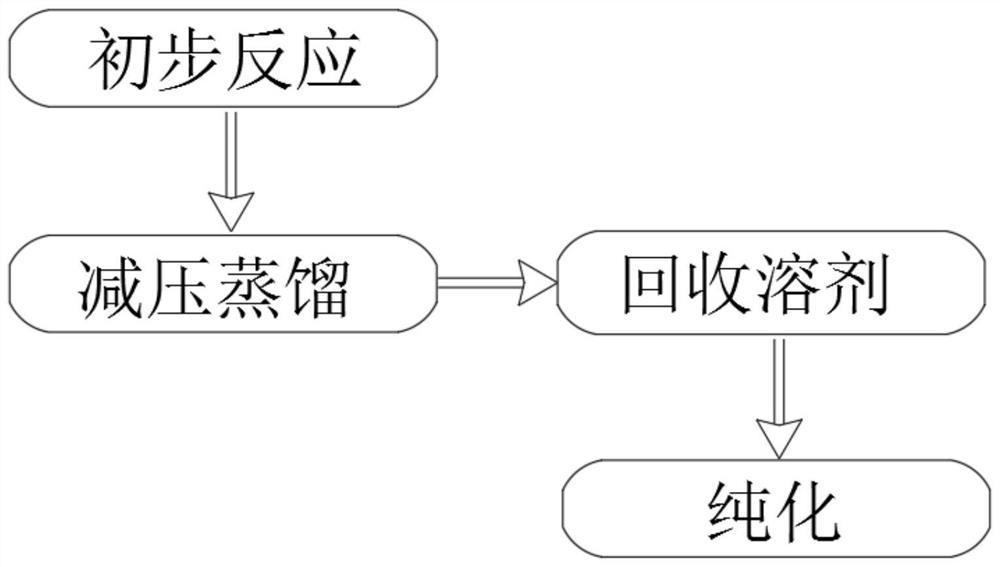
Synthesis and purification method of 4, 7-dichloroquinoline
The present invention relates to the technical field of compound synthesis, and discloses a 4, 7-dichloroquinoline synthesis purification method, which comprises: S1, primary reaction: adding 7-chloro-4-hydroxyquinoline into 300 g of toluene, sequentially adding DMF and triphenylphosphine oxide, starting stirring, internally heating to a temperature of 79-90 DEG C, slowly adding phosphorus oxychloride or a toluene solution of phosphorus oxychloride in a dropwise manner, after the dropwise adding is completed, carrying out a reaction for 2-3 h, and cooling to a room temperature to obtain the 4, 7-dichloroquinoline. Continuously reacting until the TLC monitoring reaction is finished; s2, performing reduced pressure distillation, namely performing reduced pressure distillation on the reaction liquid in the S1. The method has the advantages of simple operation, high yield, no use of any reagent in the purification process, no introduction of new impurities, no special requirements on equipment, no environmental pollution, no introduction of an organic solvent, no pollution, and easy industrial production.
Owner:南京固与生物有限公司
Method for preparing 2,3-dichloroquinoxaline derivatives in one pot
ActiveCN108191778BMild reaction conditionsSimple and fast operationOrganic chemistryPharmaceutical drugCooking methods
The invention belongs to the field of drug synthesis, and specifically relates to a new method for preparing 2,3-dichloroquinoxaline derivatives in one pot. Friendly silica gel or methanesulfonic acid is used as the catalyst, and the intermediate separation and purification steps are omitted, the operation is simple, the cost is low, the reaction conditions are mild, and it is environmentally friendly, and it is easy to industrialize large-scale production.
Owner:CHONGQING MEDICAL UNIVERSITY
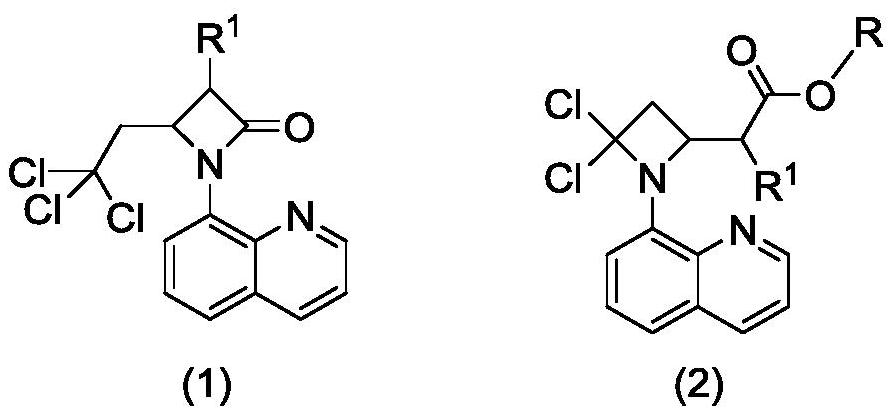
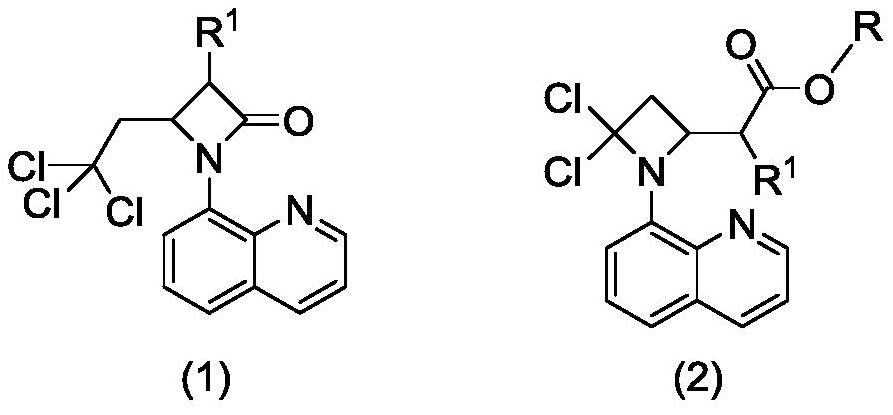
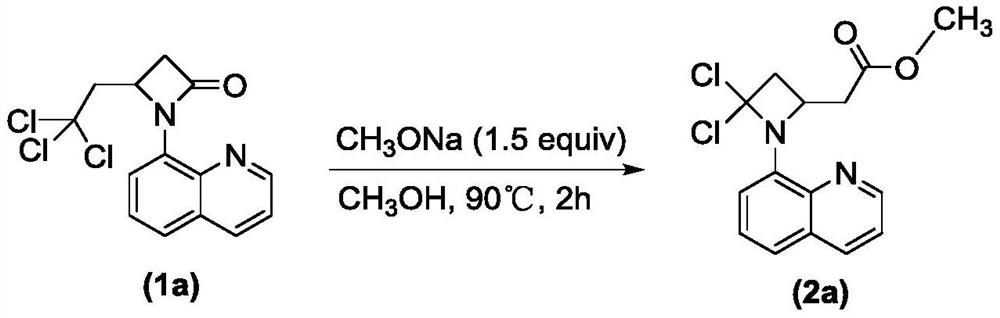
Preparation method of 2-(4, 4-dichloro-1-(8-quinolyl)-2-azacyclobutyl) carboxylic ester derivative
ActiveCN113045539ARich typeRaw materials are environmentally friendly and easy to obtainOrganic chemistryAlcoholQuinolizine
The invention relates to a preparation method of a 2-(4, 4-dichloro-1-(8-quinolyl)-2-azacyclobutyl) carboxylic ester derivative, which comprises the following steps of reacting a substituted 4-(2, 2, 2-trichloroethyl)-1-(8-quinolyl)-beta-lactam derivative, a nucleophilic reagent sodium alkoxide and a monohydric alcohol solvent at 80-110 DEG C to obtain the 2-(4, 4-dichloro-1-(8-quinolyl)-2-azacyclobutyl) carboxylic ester derivative. The raw materials are easy to obtain, environmentally friendly and various in variety, the obtained products are various in type, can be directly used and can also be used for other further reactions, the reaction conditions are mild, the process is simple, the yield is high, and the method is suitable for large-scale production.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthesis method of 3-ethyl carboxylate-4-chloro imidazole [1,5-a] quinoxaline Synthesis method of 3-ethyl carboxylate-4-chloro imidazole [1,5-a] quinoxaline](https://images-eureka.patsnap.com/patent_img/c46e330d-17f1-4136-80d2-1dd46ea0f721/2010102929546100002DEST_PATH_IMAGE005.PNG)
![Synthesis method of 3-ethyl carboxylate-4-chloro imidazole [1,5-a] quinoxaline Synthesis method of 3-ethyl carboxylate-4-chloro imidazole [1,5-a] quinoxaline](https://images-eureka.patsnap.com/patent_img/c46e330d-17f1-4136-80d2-1dd46ea0f721/2010102929546100002DEST_PATH_IMAGE007.PNG)
![Synthesis method of 3-ethyl carboxylate-4-chloro imidazole [1,5-a] quinoxaline Synthesis method of 3-ethyl carboxylate-4-chloro imidazole [1,5-a] quinoxaline](https://images-eureka.patsnap.com/patent_img/c46e330d-17f1-4136-80d2-1dd46ea0f721/2010102929546100002DEST_PATH_IMAGE009.PNG)