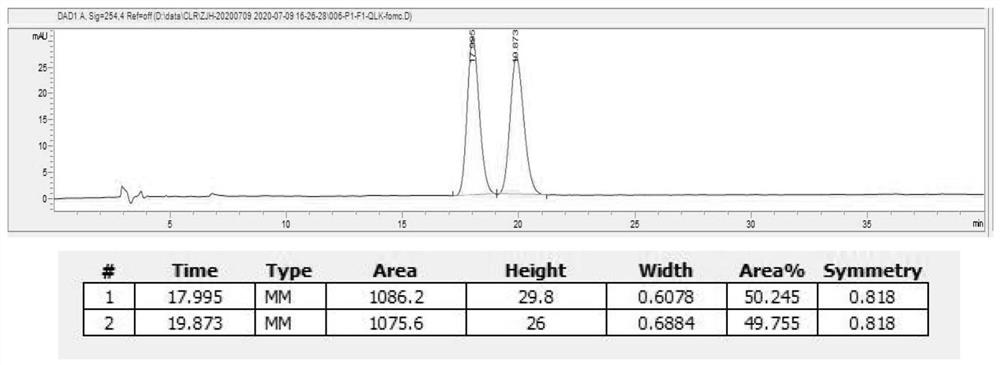
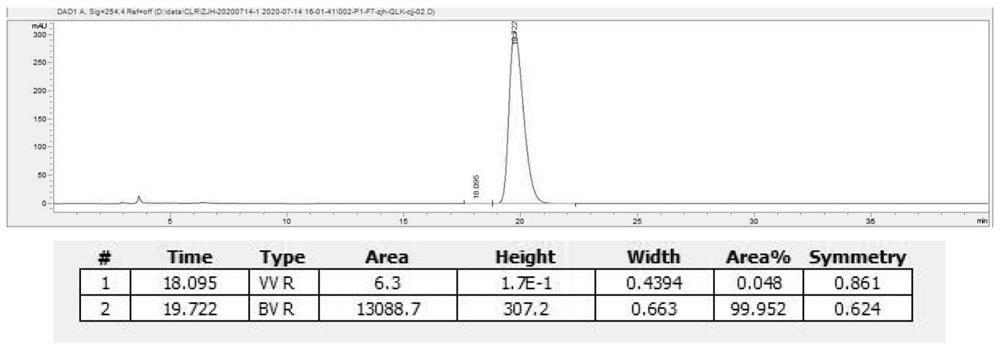
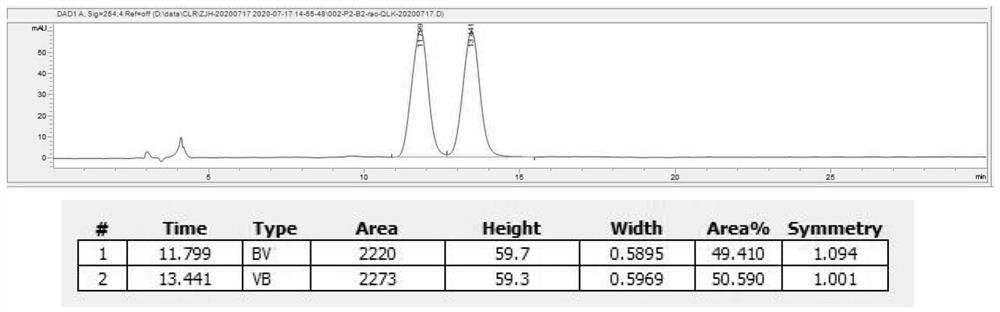
Asymmetric synthesis method of optically pure (R)/(S)-hydroxychloroquine side chain
A synthetic method and technology of hydroxychloroquine, which is applied in the field of asymmetric synthesis of optically pure /- hydroxychloroquine side chains, can solve the problems of low ee value and low yield, and achieve material cost saving, high atom economy and conversion rate and highly selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Splitting of racemic hydroxychloroquine side chains
[0040]A 500mL jacketed bottle is equipped with mechanical stirring, a reflux condenser and a thermometer. Add 80g of hydroxychloroquine side chain and 60mL of isopropanol to the jacketed bottle and turn on the mechanical stirring. Add 36.32g of (S)-mandelic acid to the bottle, Connect the bottle to an 86°C circulating water bath. When the temperature reaches about 80°C, add 360mL of toluene to the bottle after it dissolves, and continue stirring for 1-2 hours after adding toluene (internal temperature 83-86°C). After 1 to 2 hours, the temperature began to drop. When the temperature dropped to 49°C, the system began to become turbid. After adding seed crystals into the bottle, the turbidity intensified, and a white solid was precipitated. Let it cool down to room temperature naturally under stirring. After cooling down to room temperature, remove the circulating water bath and connect to a circulating cooling bath...
Embodiment 2
[0051] The preparation method of embodiment 2 (S)-hydroxychloroquine side chain
[0052] Get 84.0 g of (S)-hydroxychloroquine side-chain mandelate obtained by splitting in Example 1 and dissolve it with 200 mL of water, add 1M NaOH solution, adjust pH=11-12, add appropriate amount of sodium chloride to the water phase, and use dichloro Methane was extracted three times; the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated to give 44.8 g of a colorless oil, which was (S)-2-[(4-aminopentyl)ethylamino]ethanol ( That is, S-hydroxychloroquine side chain).
Embodiment 3
[0053] The preparation method of embodiment 3 (S)-hydroxychloroquine sulfate
[0054] The (S)-2-[(4-aminopentyl)ethylamino]ethanol obtained in Example 2 was transferred to a three-necked flask, and 56g of 4,7-dichloroquinoline and 8mL of isopropanol were weighed, heated to Stir at 130°C, react for 23 hours, stop the reaction, and cool naturally; add 2M HCl solution, adjust pH=2, wash away a small amount of unreacted 4,7-dichloroquinoline with dichloromethane; add 1M NaOH solution to the above water phase Adjust the pH to >12, extract with dichloromethane, wash the organic phase with water until the pH of the aqueous phase = 7-8, to remove residual chiral side chains. The organic phase was rotary evaporated to a brown extract with no change in quality to obtain the crude product of (S)-hydroxychloroquine free base, weighing 78.6 g.
[0055] Add the crude product of (S)-hydroxychloroquine free base to 325g of 95% ethanol (about 4 times the mass of the extract), and stir to diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com