Preparation method of hydroxypiperaquine and phosphate thereof
A technology of hydroxypiperaquine and hydroxypiperaquine phosphate, which is applied in the field of medicine, can solve the problems of low yield, seldom reported synthetic methods of hydroxypiperaquine, and inability to obtain hydroxypiperaquine, and achieves simple preparation method, low cost, and easy preparation. The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] In the present embodiment, hydroxypiperaquine and hydroxypiperaquine phosphate are prepared by the following method, comprising the following steps:
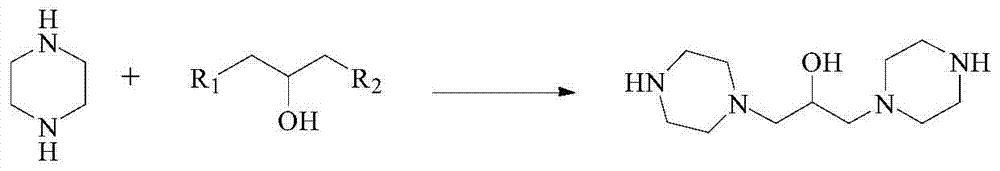
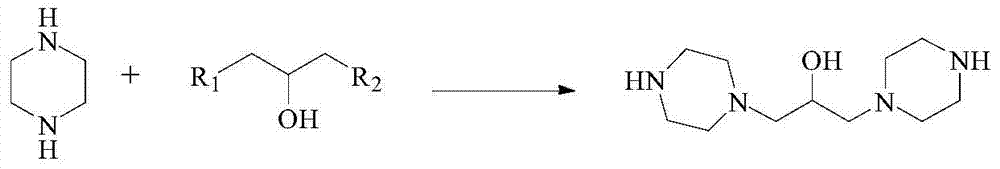
[0065] (1) Synthesis of 1,3-dipiperazinyl propanol
[0066] Add 400 g of ethanol, 129 g (1 mol) of 1,3-dichloropropanol and 173 g (2 mol) of piperazine to the reaction flask. After dissolving, slowly add 88 g (2.2 mol) of acid-binding agent sodium hydroxide, and then react at 90°C 3h. After the reaction, cool down to room temperature, remove the formed inorganic salt by filtration, and remove the solvent under reduced pressure to obtain 210 g of 1,3-dipiperazinylpropanol (92.1% yield) with a melting point of 128°C.
[0067] The proton nuclear magnetic resonance spectrum characterization result of step (1) product is: 1 H NMR (CDCl 3 )δppm: 1.54(1H), 19.1(2H), 2.37(8H), 2.46(4H), 2.65(8H).
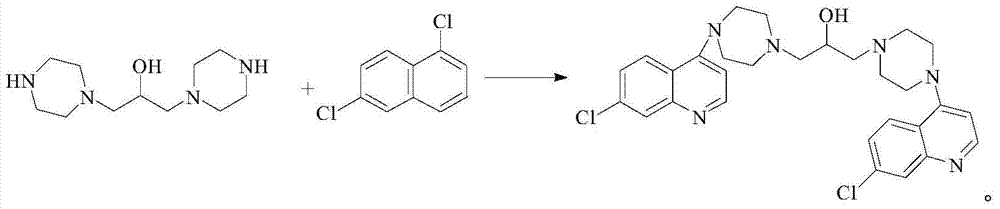
[0068] (2) synthetic hydroxypiperaquine
[0069] Add 1054g ethanol, 351.4g (1.774mol) 4,7-dichloroquinoline and 200g (0.877mol) ...
Embodiment 2
[0075] In the present embodiment, hydroxypiperaquine and hydroxypiperaquine phosphate are prepared by the following method, comprising the following steps:
[0076] (1) Synthesis of 1,3-dipiperazinyl propanol
[0077] Add 1075g water, 64.5g (0.5mol) 1,3-dichloropropanol and 215g (2.5mol) piperazine in reaction bottle, after treating to dissolve, slowly add 80g (2mol) acid-binding agent sodium hydroxide, then in 50 ℃ reaction 7h. After the reaction, cool down to room temperature, remove the formed inorganic salt by filtration, and remove the solvent under reduced pressure to obtain 104.3 g of 1,3-dipiperazinylpropanol (91.5% yield) with a melting point of 128°C.
[0078] The proton nuclear magnetic resonance spectrum characterization result of step (1) product is: 1 H NMR (CDCl 3 )δppm: 1.54(1H), 19.1(2H), 2.37(8H), 2.46(4H), 2.65(8H).
[0079] (2) synthetic hydroxypiperaquine
[0080] Add 261g of acetonitrile, 260.2g (1.314mol) of 4,7-dichloroquinoline and 100g (0.438mol)...
Embodiment 3
[0086] In the present embodiment, hydroxypiperaquine and hydroxypiperaquine phosphate are prepared by the following method, comprising the following steps:
[0087] (1) Synthesis of 1,3-dipiperazinyl propanol
[0088] Add 172g of water, 64.5g (0.5mol) of 1,3-dichloropropanol and 172g (2mol) of piperazine to the reaction flask. After dissolving, slowly add 159g (1.5mol) of acid-binding agent sodium carbonate, and then Reaction 6h. After the reaction, cool down to room temperature, remove the formed inorganic salt by filtration, and remove the solvent under reduced pressure to obtain 106.2 g of 1,3-dipiperazinylpropanol (93.2% yield) with a melting point of 128°C.
[0089] The proton nuclear magnetic resonance spectrum characterization result of step (1) product is: 1 H NMR (CDCl 3 )δppm: 1.54(1H), 19.1(2H), 2.37(8H), 2.46(4H), 2.65(8H).
[0090] (2) synthetic hydroxypiperaquine
[0091] Add 500g of propanol, 216.8g (1.095mol) of 4,7-dichloroquinoline and 100g (0.438mol) of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com