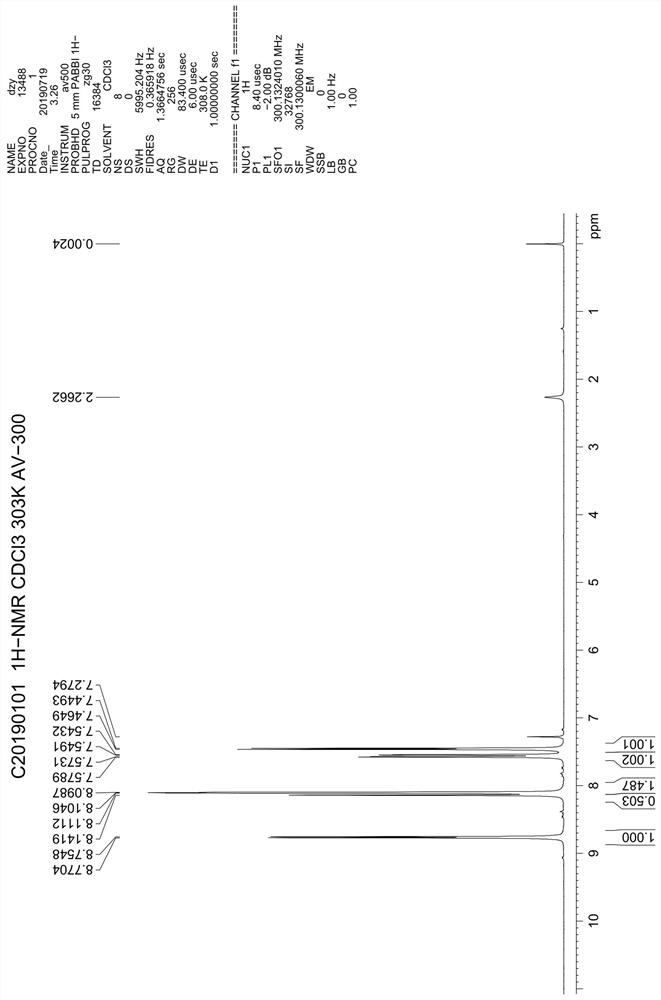
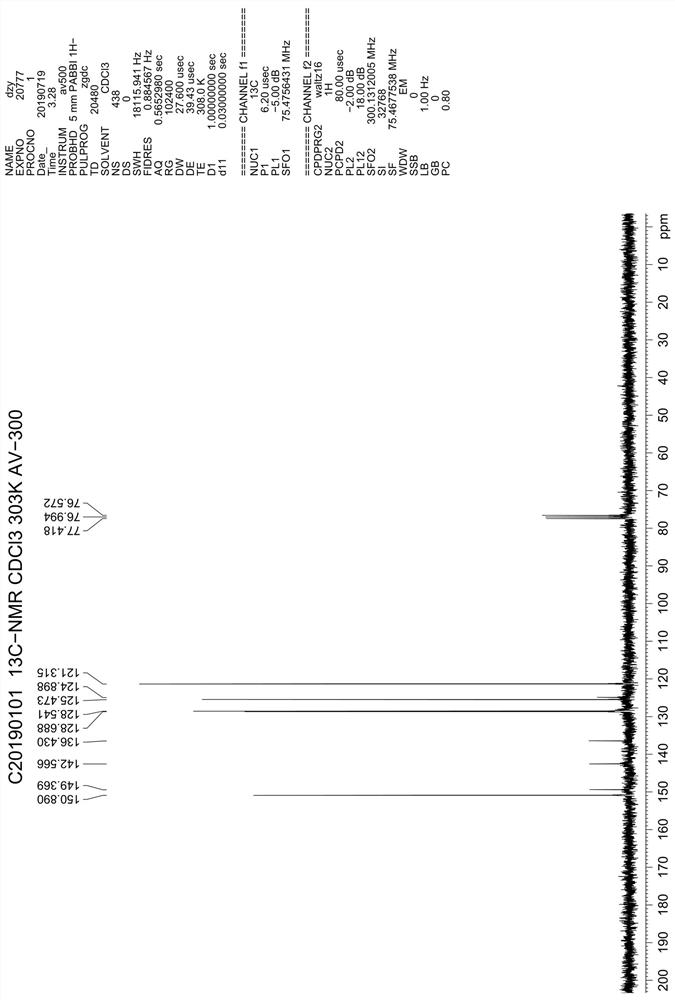
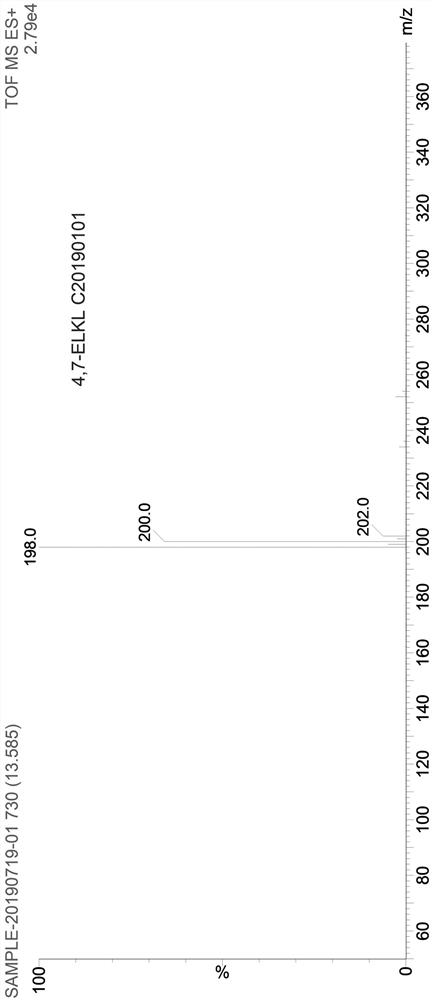
Preparation method of 4, 7-dichloroquinoline
A technology of dichloroquinoline and hydroxyquinoline, applied in the field of medicine, can solve problems such as danger and influence, and achieve the effect of quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 240g of toluene, 90g of ethoxymethylenemalonate diethyl ester, 60g of 2-amino-6-chlorobenzoic acid into a 500mL four-necked reaction flask, stir and heat up to 60°C for 6 hours, when the reaction is complete, add 12g FeCl 3 , continue to heat up and reflux for 24 hours, and when the reflux is over, evaporate toluene under reduced pressure to dryness, then add 300ml of drinking water to the reaction bottle, stir and break up, transfer the feed solution to a 1L four-necked flask, and then add 300ml of drinking water and 56g caustic soda, stir and heat up to reflux for 6 hours, cool to 50-60°C, adjust pH to about 3 with hydrochloric acid, continue to cool down to below 20°C, filter, wash the solid with water, and dry to obtain intermediate V.
[0025] Put the tide product of intermediate Ⅴ into a 500ml four-necked reaction bottle, dry fry at 250°C for 4 hours, then cool down to about 60°C, add 240g of fresh phosphorus oxychloride, heat up and reflux for 5 hours, and th...
Embodiment 2
[0027] Add 360g of xylene, 90g of diethyl ethoxymethylenemalonate, 60g of 2-amino-6-chlorobenzoic acid into a 1L four-necked reaction flask, stir and raise the temperature to about 80°C for 4 hours. After the reaction is complete, add 10g FeCl 3 , continue to heat up and reflux for 16 hours, reflux is over, distill off xylene under reduced pressure to dryness, then add 800ml of drinking water and 84g of caustic soda to the reaction bottle, stir and heat up to reflux for 2 hours, cool to 50-60°C, use Adjust the pH to about 2 with hydrochloric acid, continue to cool down to below 20°C, filter, wash the solid with water, and dry to obtain intermediate V.
[0028] Put the tide product of intermediate Ⅴ into a 500ml four-necked reaction bottle, dry-fry at 300°C for 2 hours, then cool down to about 60°C, add 480g of fresh phosphorus oxychloride, heat up and reflux for 1 hour, and then change to vacuum distillation to excess Phosphorus oxychloride, after distillation, slowly pour th...
Embodiment 3
[0030] Add 480g of chlorobenzene, 90g of ethoxymethylenemalonate diethyl ester, 60g of 2-amino-6-chlorobenzoic acid into a 1L four-necked reaction flask, stir and raise the temperature to about 70°C for 5 hours. After the reaction is complete, add 6g FeCl 3 , continue to heat up and reflux for 12 hours, reflux is over, decompress and evaporate chlorobenzene to dryness, then add 700ml of drinking water and 75g of caustic soda to the reaction bottle, stir and heat up to reflux for 4 hours, cool to 50-60°C, use Adjust the pH to about 2 with hydrochloric acid, continue to cool down to below 20°C, filter, wash the solid with water, and dry to obtain intermediate V.
[0031] Put the tide product of intermediate Ⅴ into a 500ml four-necked reaction bottle, dry fry at 270°C for 3 hours, then cool down to about 60°C, add 360g of fresh phosphorus oxychloride, heat up and reflux for 3 hours, then change to vacuum distillation to excess Phosphorus oxychloride, after distillation, slowly p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com