6,7-dichloroquinoline-5,8-dione derivative transition metal complex and its synthesis method and application
A compound and composition technology, applied in the field of medicine, can solve problems such as limitations and inactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
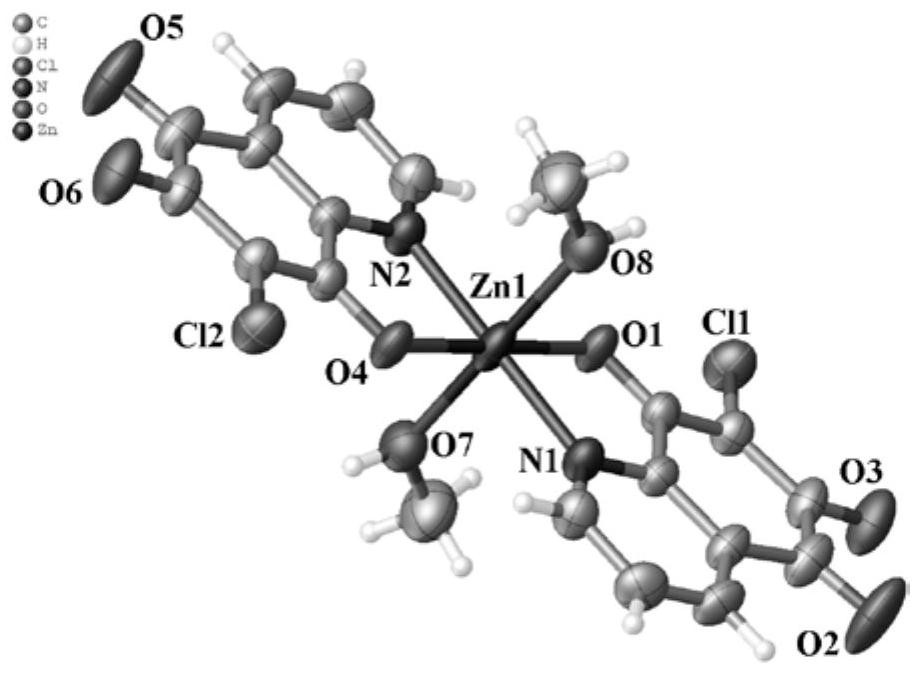
[0028] Embodiment 1: [Zn(DQ) 2 (CH 3 Oh) 2 ] (complex 1) synthesis
[0029] Weigh 2.0mmol of DQ and 1.0mmol of Zn(NO 3 ) 2 ·6H 2 O, placed in a 100.0mL high-temperature pressure-resistant tube, dissolved in 35.0mL of mixed solvent (V 甲醇 :V 二氯甲烷 =7:93), react at 65°C for 6.0h, filter the reactant, and volatilize the obtained filtrate at room temperature until its volume is 1 / 3 of the volume of the mixed solvent, a large number of reddish-brown blocky crystals are precipitated, collect the crystals , washed with methanol, and dried at 45°C to obtain the reddish-brown target complex 1. The yield was 90.0%.
[0030] The product obtained in this embodiment is characterized:
[0031] (1) X-ray single crystal diffraction:
[0032] Take a moderately sized reddish-brown bulk crystal and place it on Agilent’s SuperNova single crystal diffractometer, using graphite monochromatized Mo-K α ray for single crystal testing. The initial crystal structures of the products obtained...
Embodiment 2
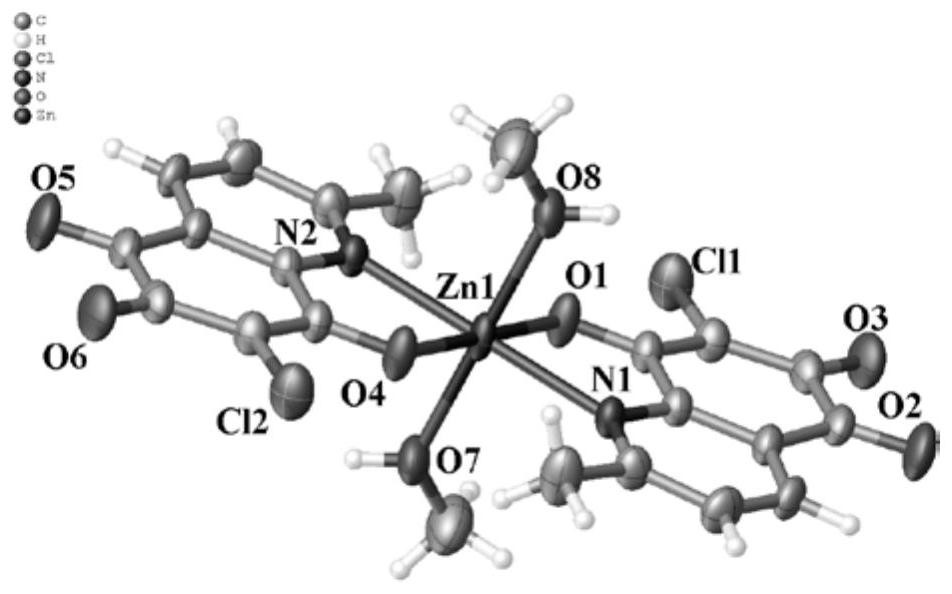
[0052] Embodiment 2: [Zn(DMQ) 2 (CH 3 Oh) 2 ] (complex 2) synthesis
[0053] Weigh 2.0mmol of DMQ and 1.0mmol of Zn(NO 3 ) 2 ·6H 2 O, placed in a 100.0mL high-temperature pressure-resistant tube, dissolved in 5.0mL of mixed solvent (V 甲醇 :V 二氯甲烷 =99:1), react at 65°C for 3.0h, filter the reactant, and volatilize the obtained filtrate at room temperature until its volume is 1 / 3 of the volume of the mixed solvent, a large number of reddish-brown block crystals are precipitated, collected The crystals were washed with methanol and dried at 45°C to obtain the reddish-brown target complex 2. The yield was 65.3%.
[0054] The product obtained in this embodiment is characterized:
[0055] (1) X-ray single crystal diffraction:
[0056] Take a moderately sized reddish-brown bulk crystal and place it on Agilent’s SuperNova single crystal diffractometer, using graphite monochromatized Mo-K α ray for single crystal testing. The initial crystal structures of the products obta...
Embodiment 3
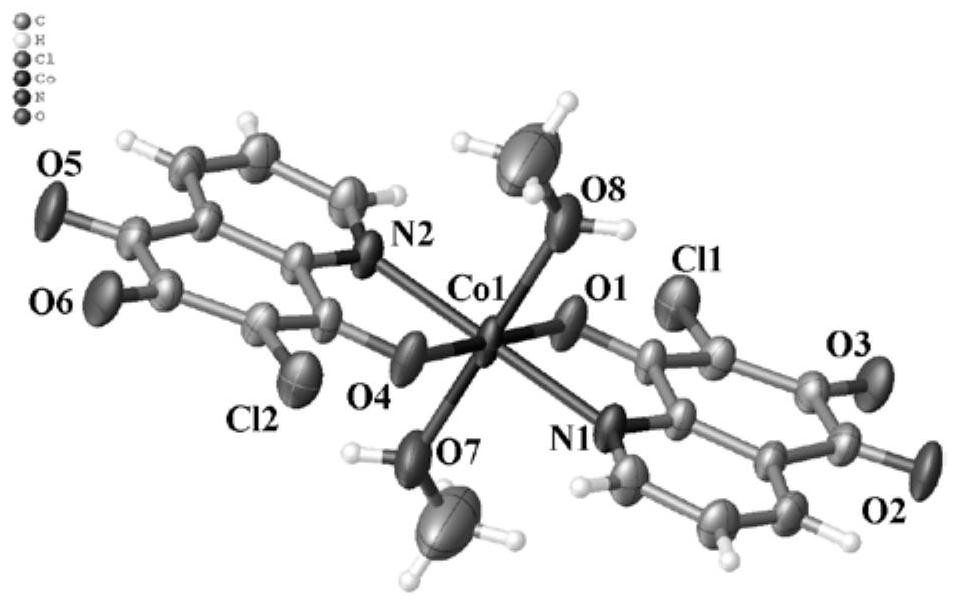
[0076] Embodiment 3: [Co(DQ) 2 (CH 3 Oh) 2 ] (complex 3) synthesis
[0077] Weigh 2.0mmol of DQ and 1.0mmol of Co(NO 3 ) 2 ·6H 2 O, placed in a 100.0mL high-temperature pressure-resistant tube, dissolved in 80.0mL of mixed solvent (V 甲醇 :V 二氯甲烷 =23:77) in the mixed solution of methanol and dichloromethane (v:v=23:77), react at 120°C for 72.0h, filter the reactant, and volatilize the obtained filtrate at room temperature until its volume is equal to that of the mixed solvent. When the volume was 1 / 3, a large number of reddish-brown blocky crystals were precipitated. The crystals were collected, washed with methanol, and dried at 45°C to obtain the reddish-brown target complex 3. The yield was 92.8%.
[0078] The product obtained in this embodiment is characterized:
[0079] (1) X-ray single crystal diffraction:
[0080] Take a moderately sized reddish-brown bulk crystal and place it on Agilent’s SuperNova single crystal diffractometer, using graphite monochromatized M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com