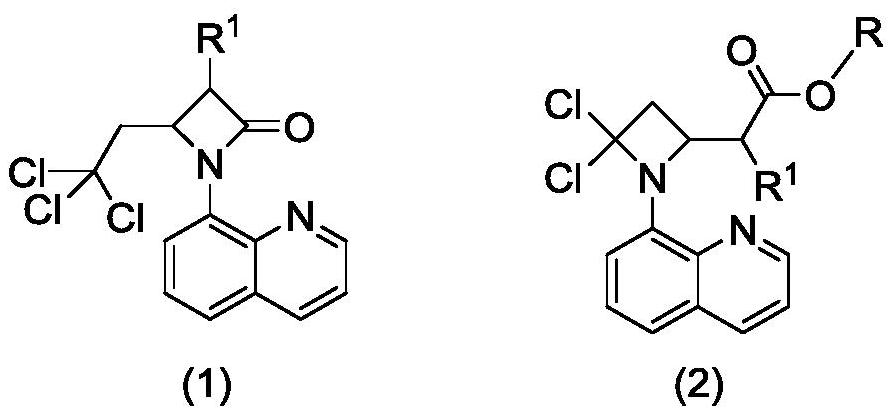
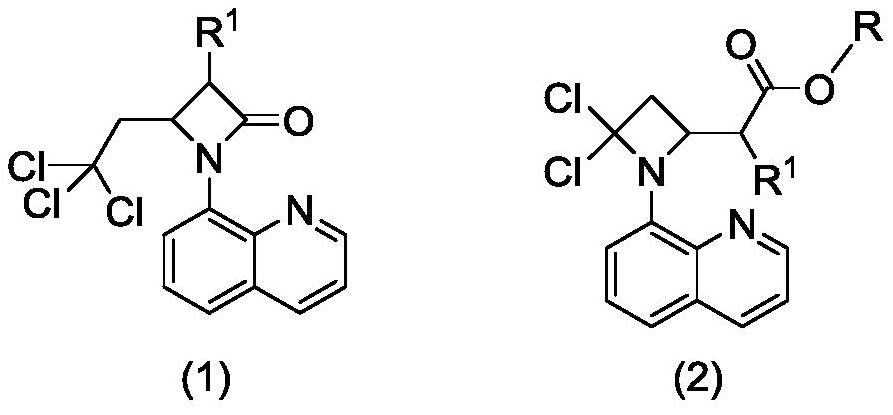
Preparation method of 2-(4, 4-dichloro-1-(8-quinolyl)-2-azacyclobutyl) carboxylic ester derivative
A technology based on azetidinyl and quinoline, applied in the field of preparation of organic compounds, can solve the problems of harsh reaction conditions, low technical yield, unfriendly environment, etc., and achieve easy-to-obtain raw materials, simple operation and post-treatment process , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
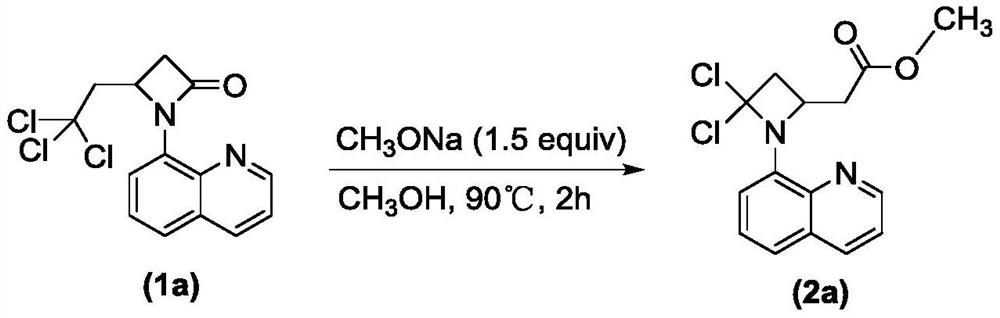
[0021] Example 1: Synthesis of 2-(4,4-dichloro-1-(8-quinolyl)-2-azetidinyl)methyl acetate
[0022]
[0023] Weigh 4-(2,2,2-trichloroethyl)-1-(8-quinolyl)-β-lactam 1a (0.066g, 0.2mmol), dissolve in sodium methoxide (0.016g, 0.03mmol) In 2mL methanol. The mixture was heated to 90°C for reaction, followed by TLC until the reaction was completely completed. After the reaction, the crude product was purified by silica gel column chromatography (petroleum ether:ethyl acetate=20:1) to obtain compound 2a. Isolated yield was 72%.
[0024] 2a: 1H NMR (400MHz, CDCl3) δ8.73 (d, J = 2.9Hz, 1H), 8.07 (d, J = 7.9Hz, 1H), 7.46–7.34 (m, 2H), 7.13 (d, J = 8.1Hz, 1H), 6.74(d, J=7.6Hz, 1H), 6.63(d, J=8.1Hz, 1H), 5.98(d, J=8.7Hz, 1H), 4.93–4.74(m, 1H) ,3.74(s,3H),2.95–2.79(m,2H).13C NMR(101MHz,CDCl3)δ170.84,147.26,142.75,138.36,136.05,131.15,128.65,127.64,122.98,121.52,115.016,106.05. 49.68, 38.94; HRMS (ESI-TOF) Calcd for C15H15Cl2N2O2[M+H]+: 325.0511, found: 325.0527.
Embodiment 2
[0025] Example 2: Synthesis of 2-(4,4-dichloro-1-(8-quinolyl)-2-azetidinyl)propionic acid methyl ester
[0026]
[0027] Weigh 3-methyl 4-(2,2,2-trichloroethyl)-1-(8-quinolyl)-β-lactam 1b (0.069g, 0.2mmol), sodium methoxide (0.016g, 0.03mmol) was dissolved in 2mL methanol. The mixture was heated to 90°C for reaction, followed by TLC until the reaction was completely completed. After the reaction, the crude product was purified by silica gel column chromatography (petroleum ether:ethyl acetate=20:1) to obtain compound 2b. Isolated yield was 72%.
[0028] 2b: 1H NMR (400MHz, CDCl3) δ8.72 (dd, J = 8.9, 3.6Hz, 1H), 8.06 (d, J = 8.2Hz, 1H), 7.43–7.34 (m, 2H), 7.09 (dd, J=17.9,8.2Hz,1H),6.84–6.53(m,2H),5.90(dd,J=36.9,9.3Hz,1H),4.71–4.28(m,1H),3.71(t,J=20.0Hz ,3H),3.25–2.88(m,1H),1.41–1.34(m,3H); HRMS(ESI-TOF) Calcd for C16H16Cl2N2O2Na[M+Na]+:361.0487,found:361.0491.
Embodiment 3
[0029] Example 3: Synthesis of 2-(4,4-dichloro-1-(8-quinolyl)-2-azetidinyl)butanoic acid methyl ester
[0030]
[0031] Weigh 3-ethyl 4-(2,2,2-trichloroethyl)-1-(8-quinolyl)-β-lactam 1c (0.071g, 0.2mmol), sodium methoxide (0.016g, 0.03mmol) was dissolved in 2mL methanol. The mixture was heated to 90°C for reaction, followed by TLC until the reaction was completely completed. After the reaction, the crude product was purified by silica gel column chromatography (petroleum ether:ethyl acetate=20:1) to obtain compound 2c. Isolated yield was 70%.
[0032] 2c:1H NMR (400MHz, CDCl3) δ8.76–8.68(m,1H),8.09–8.03(m,1H),7.43–7.33(m,2H),7.14–7.04(m,1H),6.85–6.46 (m,2H),5.88(dd,J=50.9,9.3Hz,1H),4.70–4.27(m,1H),3.73(t,J=15.9Hz,3H),3.20–2.70(m,1H), 1.98–1.69(m,2H),1.05–0.96(m,3H); HRMS(ESI-TOF) Calcd for C17H19Cl2N2O2[M+H]+:353.0824,found:353.0825.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com