Preparation method of chiral aminochloroquinoline
A technology of aminochloroquinoline and dichloroquinoline, applied in the field of medicine, can solve problems such as few chloroquine synthesis methods, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
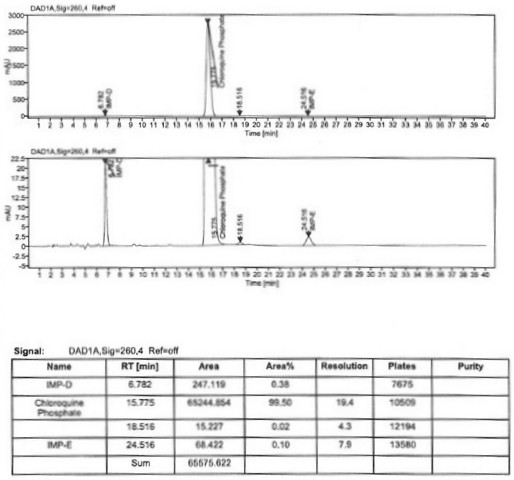
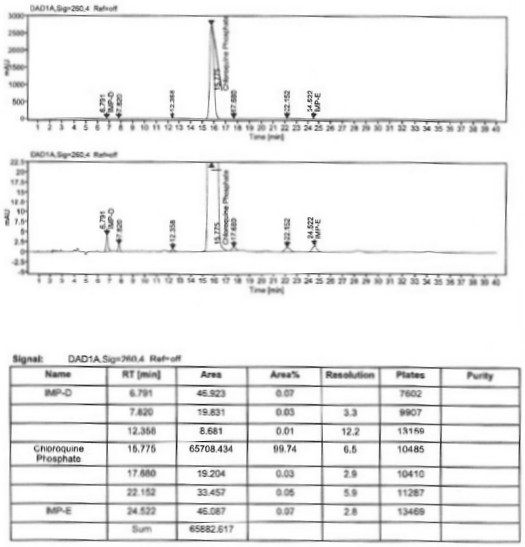
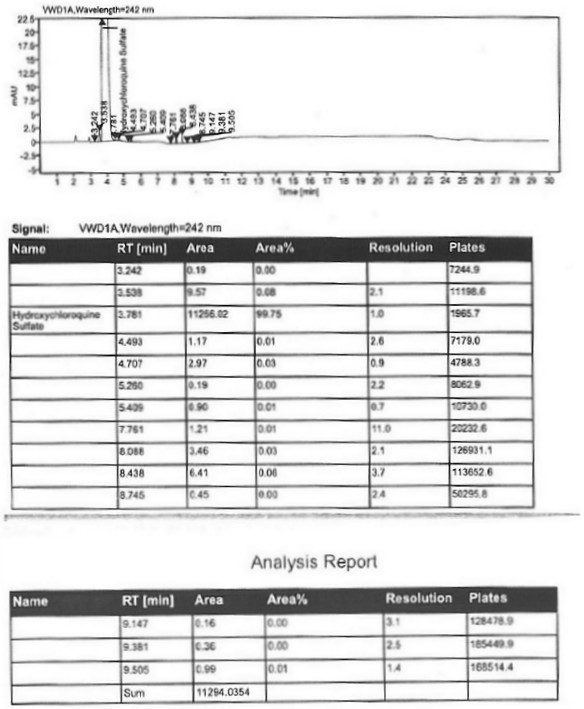
Image
Examples
Embodiment 1
[0032] The reaction formula is as follows:
[0033] 1. Resolution of 2-amino-5-diethylaminopentane:
[0034]
[0035] 2. Enantiomer synthesis:
[0036]
[0037] 1. Resolution of 2-amino-5-diethylaminopentane:
[0038] a. 80g of 2-amino-5-diethylaminopentane, 0.51mol, add 40ml of isopropanol, heat to dissolve, add 41g of D-mandelic acid, 0.27mol, stir for 6 hours, filter with suction, keep the filtrate for use, and collect off-white solid . Vacuum drying at 60-65°C yielded 67g of L-mandelic acid salt;
[0039] b. Add 41 g of L-mandelic acid (0.27 mol) to the filtrate of the previous step, stir at room temperature for 10 hours, filter, and collect an off-white solid. Vacuum drying at 60-65°C yielded 62 g of D-mandelic acid salt.
[0040] 2. Preparation of enantiomeric salts:
[0041] a. 67g of the L-mandelate prepared in the above 1(a), add 150g of purified water, add 20% sodium hydroxide to neutralize to PH = 11, add 500g of dichloromethane, stir for 30min, separate...
Embodiment 2
[0045] 1. Resolution of 2-amino-5-diethylaminopentane:
[0046]a. 80g of 2-amino-5-diethylaminopentane, 0.51mol, add 45ml of isopropanol, heat to dissolve, add 43g of D-mandelic acid, 0.28mol, stir for 7 hours, filter with suction, keep the filtrate for use, and collect off-white solid . Vacuum drying at 60-65°C yielded 68.3 g of L-mandelic acid salt;
[0047] b. Add 43g of L-mandelic acid (0.28mol) to the filtrate of the previous step, stir at room temperature for 10 hours, filter, and collect an off-white solid. Vacuum drying at 60-65°C yielded 63.4 g of D-mandelic acid salt;
[0048] 2. Preparation of enantiomeric salts:
[0049] a. Add 68.3g of L-mandelate prepared in the above 1(a), add 155g of purified water, add 25% sodium hydroxide to neutralize to PH=11, add 550g of dichloromethane, stir for 30min, separate layers, concentrate under reduced pressure for two Chloromethane, to get the side chain left-handed free base.
[0050] b. Add 43.6 g (0.22 mol) of 4,7-dichlo...
Embodiment 3
[0053] 1. Resolution of 2-amino-5-diethylaminopentane:
[0054] a. 80g 2-amino-5-diethylaminopentane 0.51mol), add 40ml isopropanol, heat to dissolve, add 60g D-camphorsulfonic acid, 0.27mol, stir for 6 hours, filter with suction, keep the filtrate for use, collect off-white solid. Vacuum drying at 60-65°C to obtain 85g of levocamphorsulfonate;
[0055] b. Add 60 g of L-camphorsulfonic acid (0.26 mol) to the filtrate of the previous step, stir at room temperature for 10 hours, filter, and collect an off-white solid. Vacuum drying at 60-65°C to obtain 80g of D-camphorsulfonate;
[0056] 2. Preparation of enantiomeric salts:
[0057] a. Add 85 g of the levocamphorsulfonate prepared in the above 1 (a), add 180 g of purified water, add 20% sodium hydroxide to neutralize to PH=11, add 500 g of dichloromethane, stir for 30 min, separate layers, and concentrate under reduced pressure. Chloromethane, to get the side chain left-handed free base.
[0058] b. Add 42 g (0.21 mol) of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com