Patents
Literature
45 results about "Chloroquinocin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
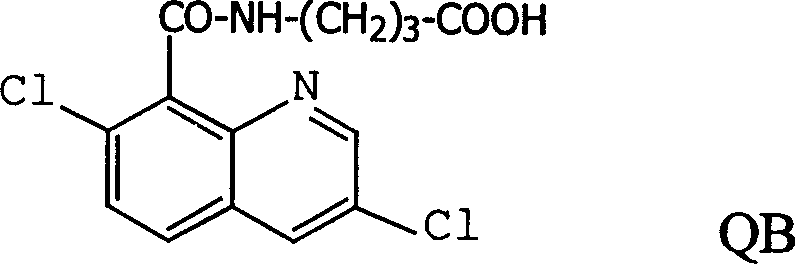
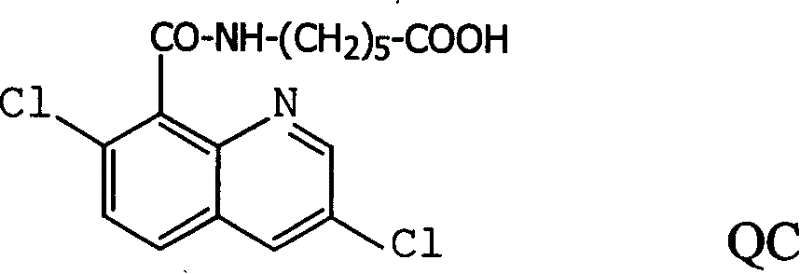
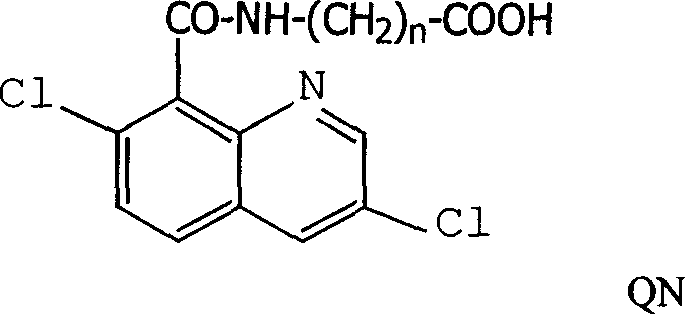
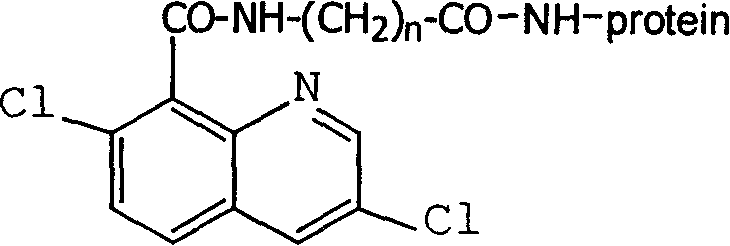
Production method and use for dichloro quinolinic acid artificial hapten, artificial antigen and specific antibody
InactiveCN1569835AEasy to handleFast and accurate analysis and detectionImmunoglobulinsTesting foodQuinolineCarboxylic acid
The invention discloses a process for preparing Quinclorac artificial semiantigen, artificial antigen, specific antibody and use thereof, wherein the preparation comprises, subjecting the dichloroquine (3,7-dichlorine-8-quinoline carboxylic acid) to sulfoxide chlorinated acylation, reacting with reanal and aminocaproic acid, thus obtaining semiantigen 4-(3,7-dichlorine-8-quinolineformyl) reanal or 6-(3,7-dichlorine-8-quinolineformyl) aminocaproic acid (QB or QC).
Owner:ZHEJIANG UNIV
Quinoline fluorescent compound, preparation method and application thereof
ActiveCN110092752ALarge displacement valueHigh quantum yieldOrganic chemistryFluorescence/phosphorescenceQuinolineOptical brightener
The invention discloses a quinoline fluorescent compound and a preparation method thereof, wherein the structural formula of the compound is as shown in the specification, X is Cl or OH; and R is anyone of CHO, CH2OH, COOH, CN and CH = NOH. The compound is obtained by taking m-diethylaminoacetanilide as a raw material to carry out Vilsmeier-Hacck reaction to obtain 2-chloroquinoline-3-formaldehyde, and subsequently obtaining a series of quinoline derivatives. The reaction conditions are mild and the operation is simple. The series of organic materials are synthesized through mutual conversionamong groups, and the absorption spectrum and the emission spectrum of the compound can be adjusted. At the same time, the disclosed compounds all have large stroke' s shift value and fluorescence quantum yield, and most of the compounds can also emit light in solid state, and can be used to develop fluorescent probes, blue luminescent materials and fluorescent whitening agents.
Owner:CHINA THREE GORGES UNIV
Carbonyl reductase gene, codase, vector, strain and application of gene
The invention discloses a carbonyl reductase derived from Rhodosporidium toruloides ZJB14212, a gene, a vector, a strain and an application of the carbonyl reductase in the preparation of chiral drug intermediates. The above carbonyl reductase can biologically catalyze preparation of highly optically pure (S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)propionamide, (S)-N-methyl-3-hydroxy-3-(2-thienyl)propionamide, (R)-3-chloro-1-(2-thienyl)1-propanol, ethyl (S)-3-hydroxy-3-(2-thienyl)propanoate, (S)-3-hydroxy-3-(2-thienyl)propionitrile, tert-butyl 6-chloro-(3R,5S)-dihydroxyhexanoate, (4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxyvaleryl]-4-phenyl-1,3-oxazolidine-2-one and methyl [S-(E)]-2-[3-[3-[2-(7-chloro-2-quinolyl)vinyl]phenyl]-3-hydroxypropyl]benzoate.
Owner:ZHEJIANG UNIV OF TECH
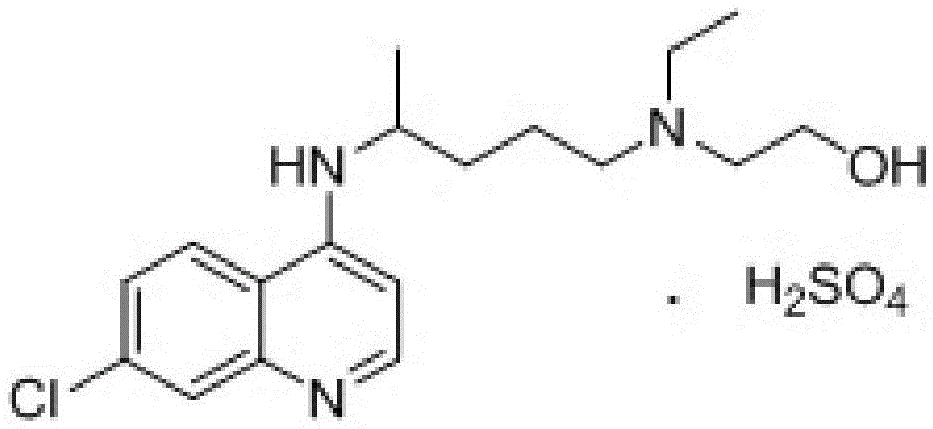
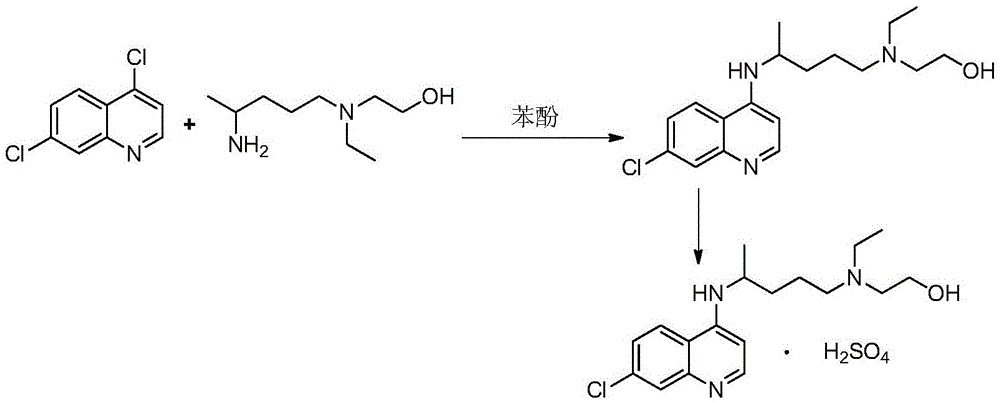
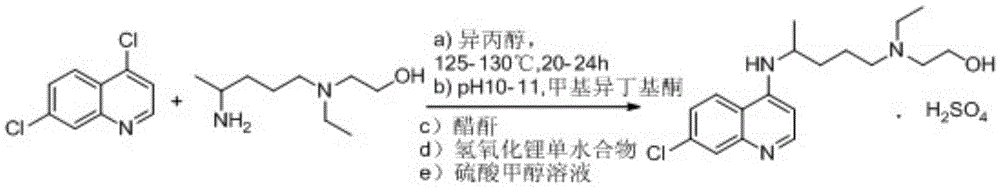
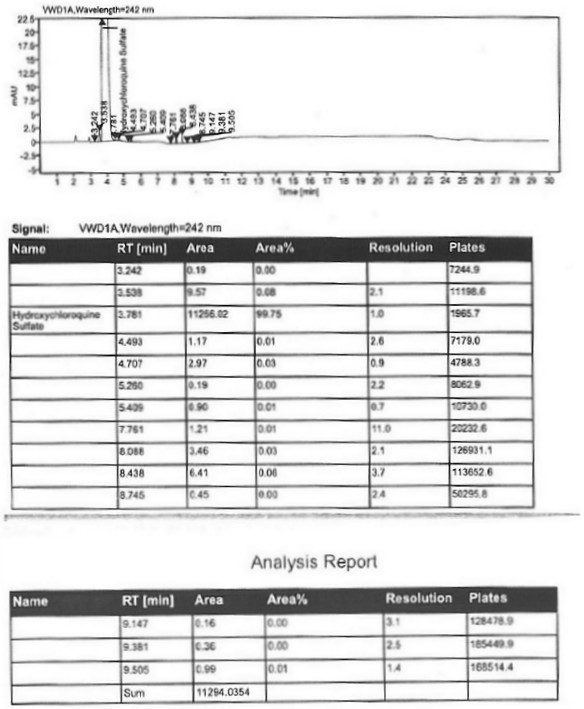
A kind of industrialized preparation method of hydroxychloroquine sulfate
The invention provides an industrial production method for hydroxychloroquine sulfate, which includes the following steps: enabling 4.7-dichloroquinoxaline and 5-(N-ethyl-N-ethoxyl)-2-amino pentane to react under gas shield for 13-24 h at a gradually increased temperature of 120-130 DEG C to obtain hydroxychloroquine; preparing the hydroxychloroquine sulfate after the reaction between the hydroxychloroquine and an alcohol sulfate solution at the temperature of 20-30 DEG C. According to the method, the yield of the obtained crude product of the hydroxychloroquine is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate HPLC is not smaller than 99.5%, the yield of single impurity is not larger than 0.1%, so that requirements of United States Pharmacopeia is met; the novel method is simple in procedure, is environment-friendly and easy in industrial production.
Owner:WUHAN WUYAO PHARMA
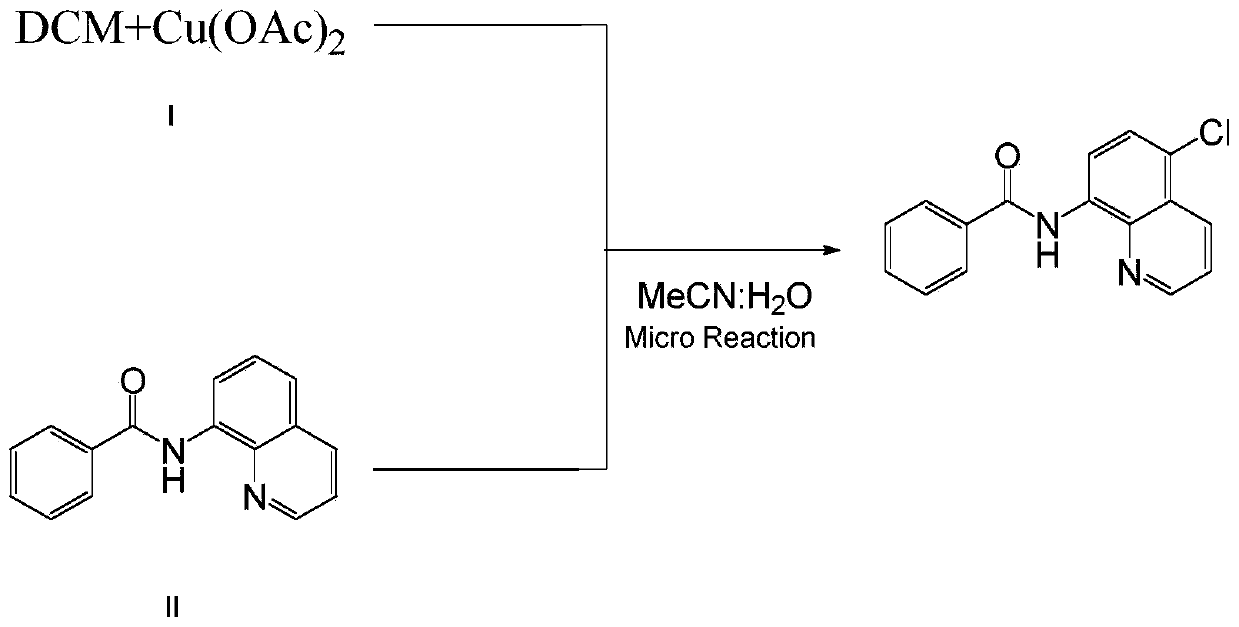
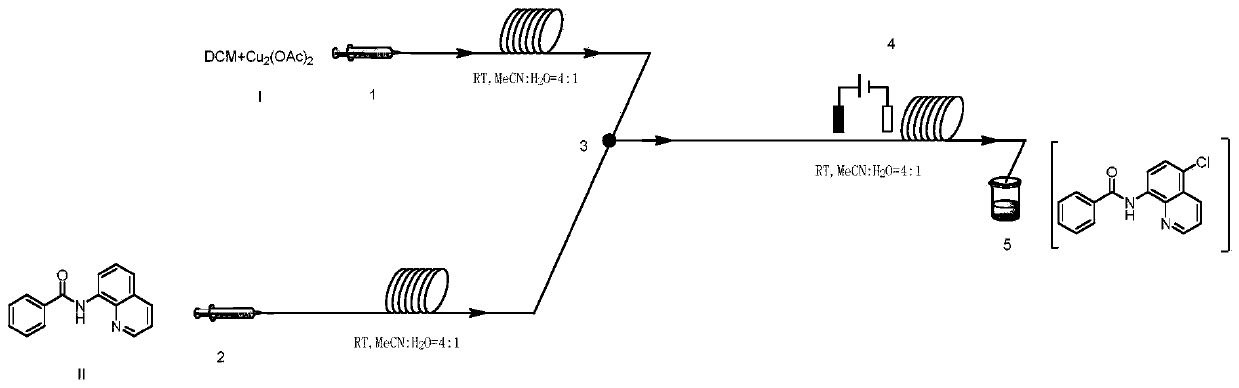
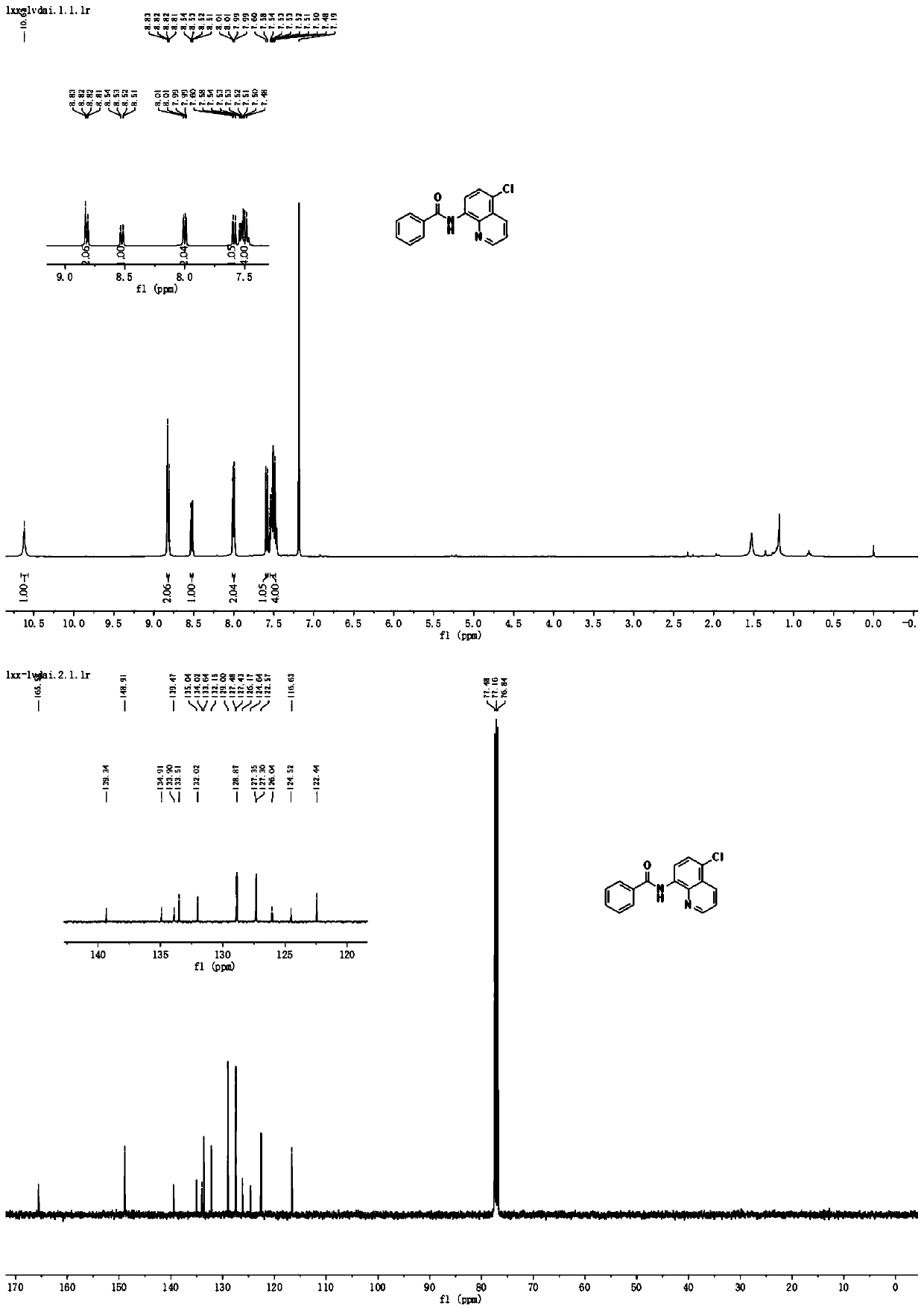
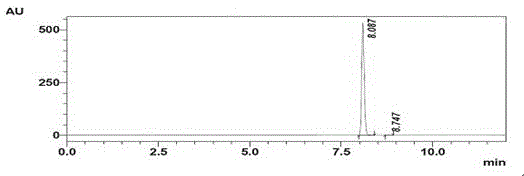
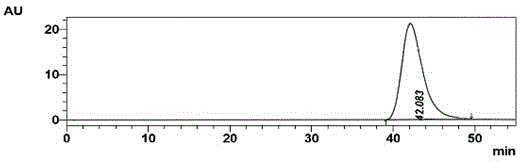
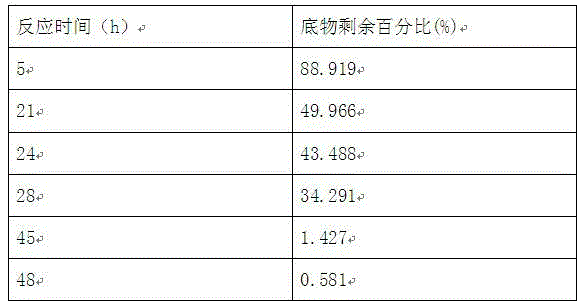
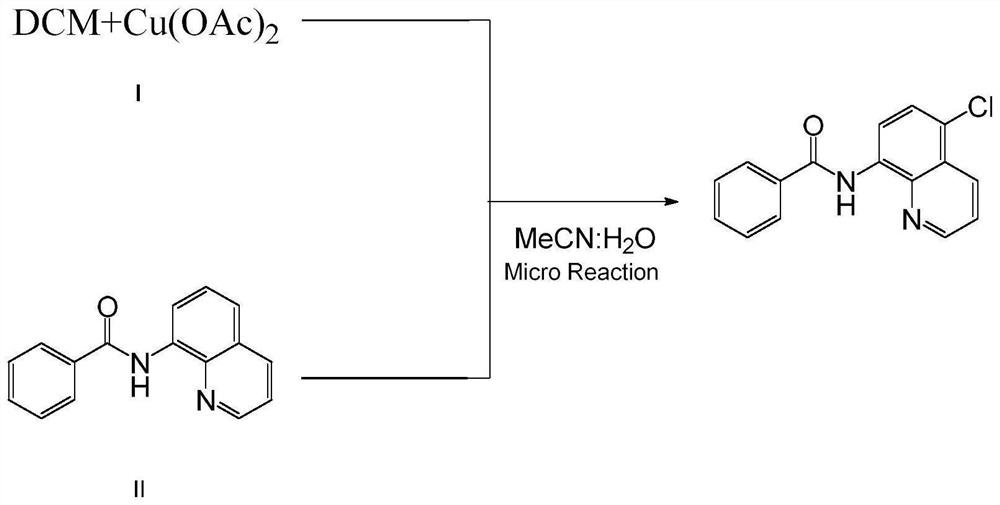
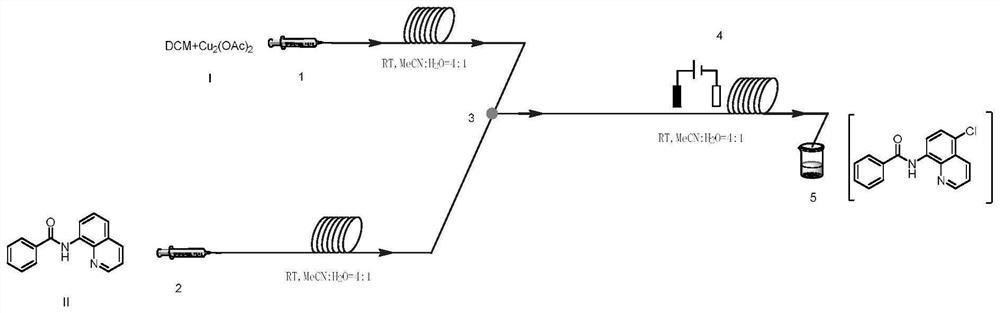
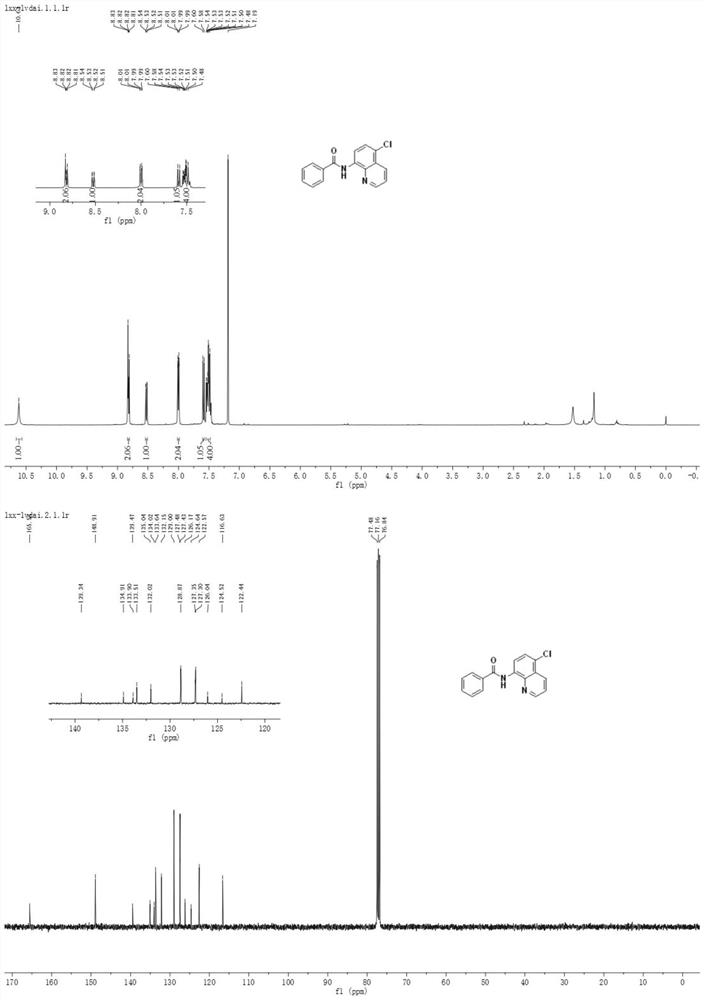
Method for preparing N-(5-chloro-8-quinolyl)benzamide compound by adopting electrochemical micro-channel reaction device
ActiveCN111519204AEasy to operateAvoid disadvantagesCellsElectrolytic organic productionPhenacylQuinoline
The invention discloses a method for preparing an N-(5-chloro-8-quinolyl)benzamide compound by adopting an electrochemical micro-channel reaction device, which comprises the following steps: dissolving an 8-(benzoylamino)quinoline compound in a first organic solvent to prepare a reaction solution A; dissolving dichloromethane and copper acetate in a second organic solvent, and preparing a reactionsolution B; respectively pumping the reaction solution A and the reaction solution B into a micro mixer of an electrochemical micro-channel reaction device at the same time for mixing, and then enabling the mixture to flow into a micro-reactor for reaction, thereby obtaining the product. Compared with the prior art, the method has the advantages that a traditional oxidizing agent is not needed, and a new method for electrocatalytically and selectively preparing the chloroquinoline compound by taking dichloromethane as a chlorination reagent through a micro-flow field is creatively developed;meanwhile, a micro-channel reaction device is utilized, so that the reaction time is greatly shortened, the reaction conversion rate is increased, and the yield is remarkably increased and reaches 85%.
Owner:NANJING UNIV OF TECH
Preparation method of chiral aminochloroquinoline
The invention discloses a preparation method of chiral aminochloroquinoline, which comprises the following steps: splitting a chiral side chain, preparing enantiomer salt, splitting the side chain andchiral acid crystals to obtain chiral acid salt, neutralizing the chiral acid salt to obtain free basic groups, and reacting the free basic groups with 4, 7-dichloroquinoline to obtain chiral aminochloroquinoline. The yield of the chiral aminochloroquinoline obtained by the method is high, and the purity reaches 99.7%.
Owner:珠海润都制药股份有限公司 +1
Synthesis method of 4,7-dichloroquinoline
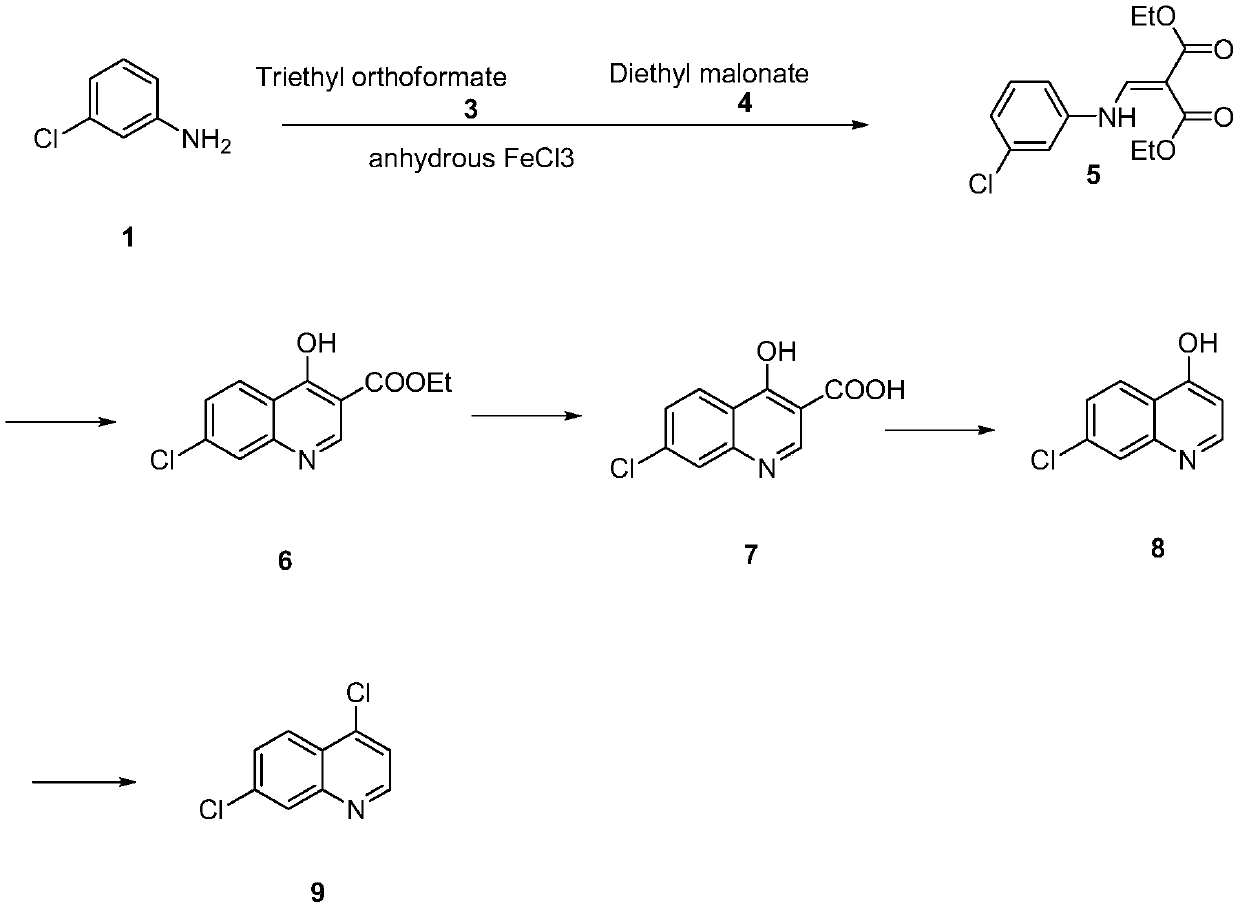
The invention discloses a synthesis method of 4,7-dichloroquinoline. The synthesis method is characterized by comprising the following steps: synthesizing 7-chloro-4-hydroxylquinoline-3-carboxylic acid by using a one-pot method, and carrying out decarboxylation and chlorination on the 7-chloro-4-hydroxylquinoline-3-carboxylic acid to obtain 4,7-dichloroquinoline. The step of synthesizing the 7-chloro-4-hydroxylquinoline-3-carboxylic acid by the one-pot method comprises the following sub-steps: with m-chloroaniline, triethyl orthoformate or trimethyl orthoformate and diethyl malonate as raw materials, carrying out condensation under the catalysis of anhydrous ferric trichloride to obtain diethyl 2-[[(3-chlorophenyl)amino]methylene]malonate, directly adding a condensation reaction solution into an organic solvent, carrying out heating cyclization to obtain 7-chloro-4-hydroxylquinoline-3-carboxylic acid ethyl ester, and after the cyclization reaction is completed, adding sodium hydroxidefor hydrolysis to obtain 7-chloro-4-hydroxylquinoline-3-carboxylic acid. Although the whole process comprises five reactions, intermediate products are good enough in purity and can be directly synthesized into a target product without purification, so operation is easy and convenient and industrialization is facilitated; and raw materials are easily available, and pollution is small.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
Preparation method of 4, 7-dichloroquinoline
ActiveCN112194621AGuaranteed qualityHigh yieldOrganic chemistryBenzoic acidDiethyl ethoxymethylenemalonate
The invention relates to a preparation method of 4, 7-dichloroquinoline in the technical field of medicines, and the method comprises the following steps of: carrying out condensation reaction on 2-amino-6-chlorobenzoic acid (I) serving as an initial raw material and diethyl ethoxymethylenemalonate (II) in an organic solvent to obtain (III), and carrying out cyclizing, hydrolyzing and acidifying on (III) to obtain hydroxyquinoline dicarboxylic acid (V). performing high-temperature decarboxylation on V to obtain hydroxyquinoline (VI), and chlorinating VI with phosphorus oxychloride to obtain 4,7-dichloroquinoline. The 4, 7-dichloroquinoline prepared by the method disclosed by the invention does not contain isomer 4, 5-dichloroquinoline, and the temperature of the condensation reaction is low under the catalysis of the catalyst, so that the reaction yield is improved, the generation of impurities is avoided, the energy consumption is reduced, and the economic benefit of the product is improved.
Owner:JIANGSU TIANHE PHARMA CO LTD
Chromeno[3',4':5,6]pyrano[2,3-b]quinoline derivative, preparation method and application thereof
InactiveCN107098914AGood antitumor activityGood in vitro inhibition of proliferationOrganic chemistryAntineoplastic agentsHepatoma cell lineQuinoline
A chromeno[3',4':5,6]pyrano[2,3-b]quinoline derivative is represented as the structure formula (I), wherein R1 is any one from H, CH3O, Cl or (CH3)3C; R2 is H or CH3; and R3 is H, CH3 or Ph. The derivative is prepared through one step with 2-chloroquinoline-3-formaldehyde or a derivative thereof, malononitrile and 4-hydroxycoumarin as raw materials, which are simple and are easy to obtain. The method has mild conditions and reaches 75% in total yield. A pharmacological test proves that the compound has excellent in-vitro proliferation inhibiting effect to human hepatoma cell line HepG2, so that the compound can inhibit growth of tumor cells and can be applied to preparation of antitumor medicines.
Owner:JIANGSU UNIV OF TECH
A kind of processing technology of non-hormonal anti-inflammatory drug intermediate
Owner:NANTONG ZHENGDA AGROCHEM
A method for preparing n-(5-chloro-8-quinolyl) benzamides using an electrochemical microchannel reaction device
ActiveCN111519204BEasy to operateAvoid disadvantagesCellsElectrolytic organic productionPhenacylQuinoline
The invention discloses a method for preparing N-(5-chloro-8-quinolyl) benzamide compounds by using an electrochemical microchannel reaction device. The 8-(benzoylamino) quinoline compounds are dissolved in The first organic solvent is made into reaction solution A; dichloromethane and copper acetate are dissolved in the second organic solvent to make reaction solution B; reaction solution A and reaction solution B are pumped into the electrochemical microchannel reaction device simultaneously respectively Mix in a micro-mixer, then flow into a micro-reactor for reaction, and obtain the product. Compared with the prior art, the present invention does not need traditional oxidants, and creatively develops a new method for the electrocatalytic selective preparation of chloroquinolines using dichloromethane as a chlorination reagent microflow field; at the same time, the present invention utilizes The microchannel reaction device greatly shortens the reaction time, improves the reaction conversion rate, and significantly increases the yield to 85%.
Owner:NANJING TECH UNIV
Selective enrichment media and uses thereof
ActiveUS10619130B1Preventing undesirable false positive responseBacteriaMicrobiological testing/measurementBiotechnologyBenzoic acid
Selective enrichment media and methods for selectively growing and detecting Salmonella spp. and / or Shiga toxin-producing E. coli. The media may comprise a carbon and nitrogen source, an inorganic salt, a fermentable sugar, one or more selective agents, and an efflux pump inhibitor. Various selective agents include sulfa drugs, surfactants, aminocoumarins, cycloheximide, supravital stains, ascorbic acid, bromobenzoic acid, myricetin, nitrofurantoin, rifamycins, polyketides, and oxazolidinones. Various efflux pump inhibitors include arylpiperazines, such as 1-(1-naphthylmethyl)piperazine, and quinoline derivatives, such as 4-chloroquinoline. Methods of selectively growing and detecting Salmonella and / or Shiga toxin-producing E. coli are provided.
Owner:PARADIGM DIAGNOSTICS
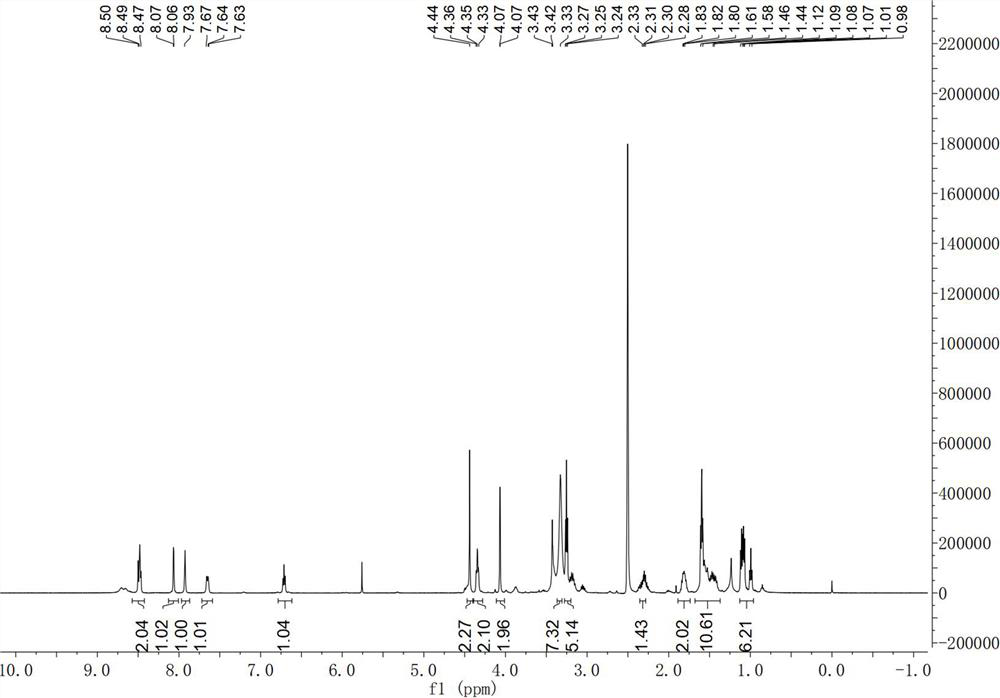
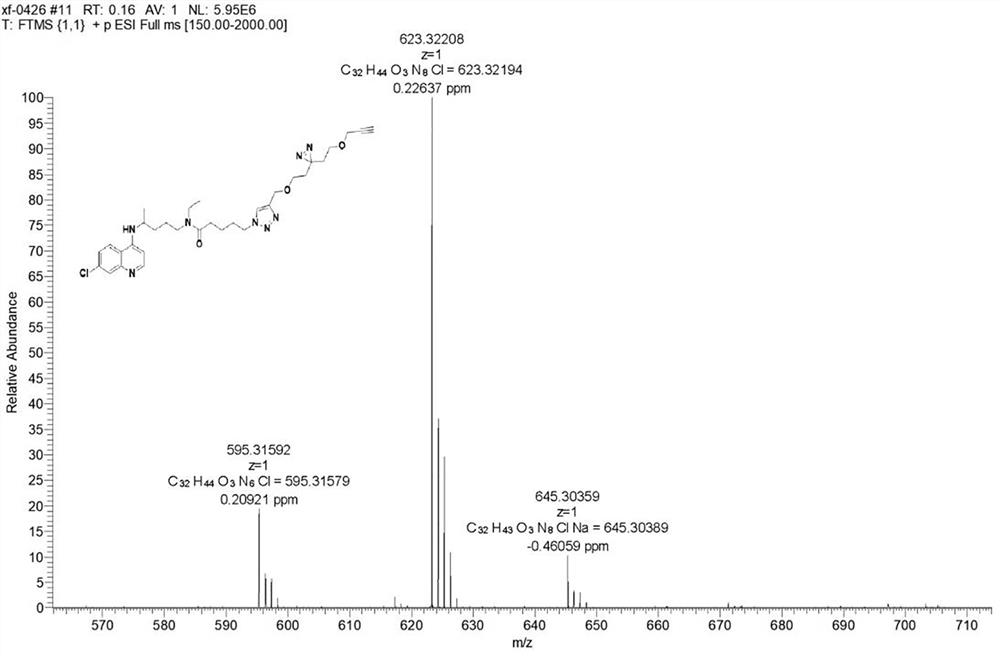
Chloroquine photoaffinity molecular probe and preparation method and application thereof
ActiveCN114560847BRealize visualizationConducive to enrichment and bindingOrganic active ingredientsOrganic chemistryProtein targetEthyl group
The invention relates to the technical field of organic chemistry, in particular to a chloroquine photoaffinity molecular probe and a preparation method and application thereof. The provided preparation method takes 1,4-dibromopentane as a raw material, and obtains N-(4-((7-chloroquine through a series of nucleophilic substitution, hydrogenation reaction, reduction reaction, amidation reaction, click reaction and the like. olin-4-yl)amino)pentyl)-N-ethyl-5-(4-((2-(3-(2-(prop-2-alkyn-1-propoxy)ethyl)-3H -Diazine-3-yl)ethoxy)methyl)-1H-1,2,3-triazol-1-yl)pentanediamine, i.e. chloroquine photoaffinity molecular probe; On the basis of , the structure was modified, and the photo-affinity diazide and alkynyl groups were introduced to obtain the probe. To achieve the purpose of visualization, enrichment and identification of target proteins, the obtained chloroquine photoaffinity molecular probe has a wide range of applications.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
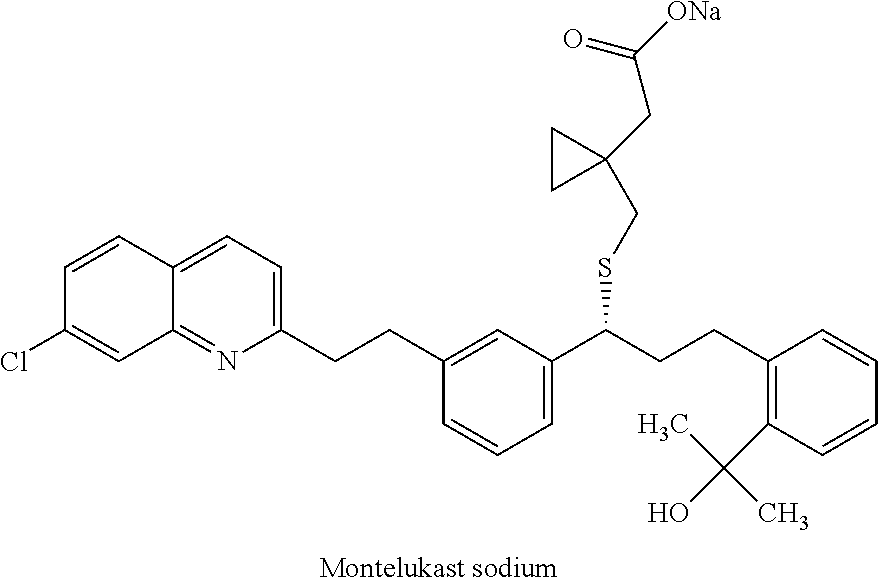
Process for preparing leukotrienes antagonist
The present invention relates to a process for the preparation of a compound of formula (I) or a sodium salt thereofwherein HET is 7-chloroquinolin-2-yl or 6,7-difluoroquinolin-2-yl, which comprises: reacting the dilithium dianion of 1-(mercapto-methyl)cyclopropaneacetic acid with a compound of formula (II)wherein HET is as defined above and L is arylsulfonyl or alkylsulfonyl. The invention further provides the dicyclohexylamine salt of a compound of formula (I).
Owner:SCHERING AG
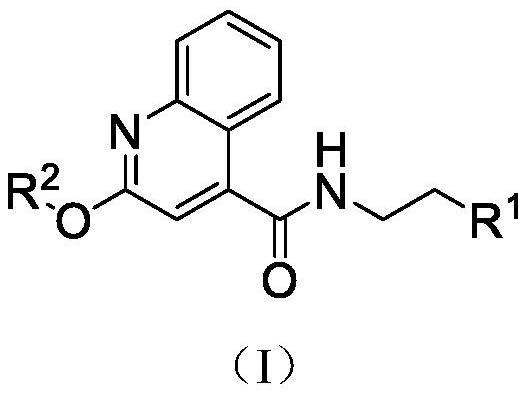
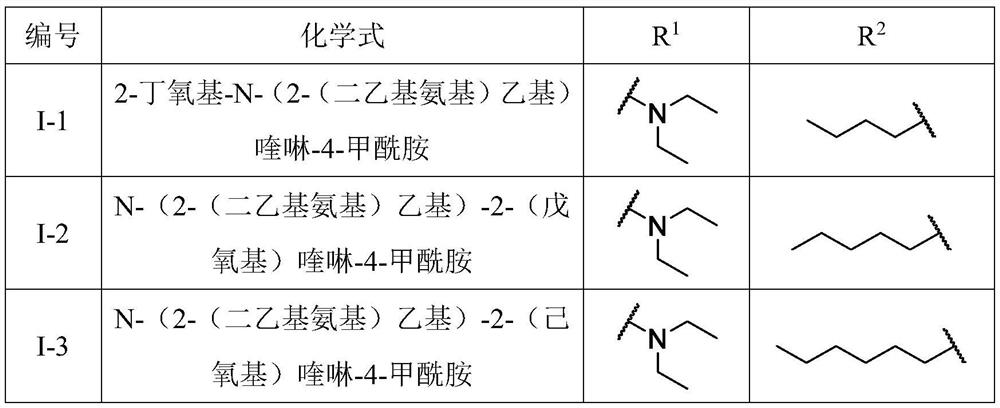
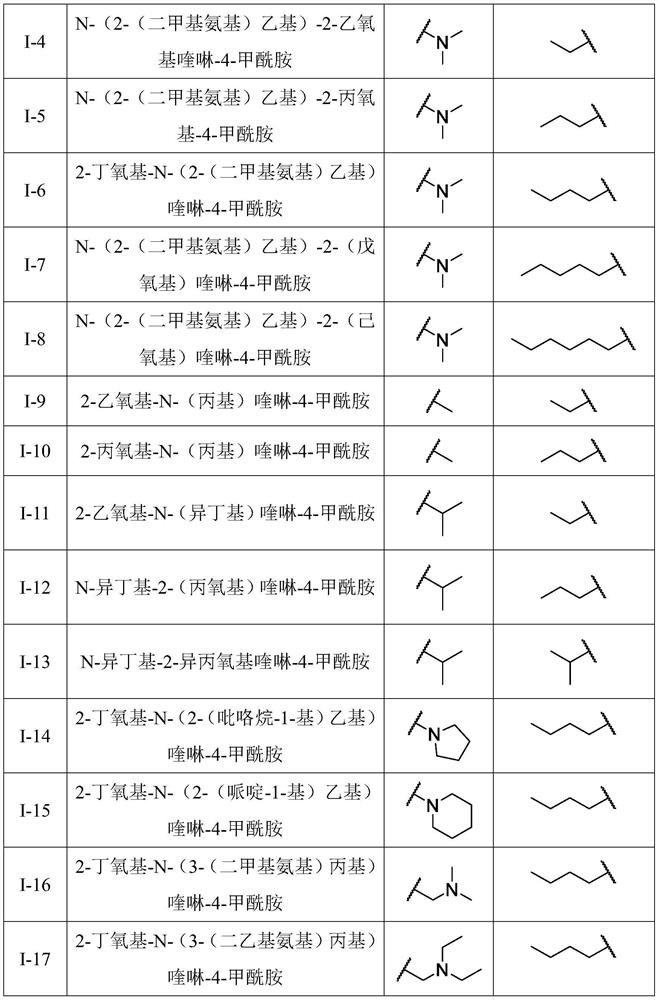
A kind of quinoline carboxamide compound and its preparation method and anti-enterovirus 71 application
Owner:WUHAN UNIV
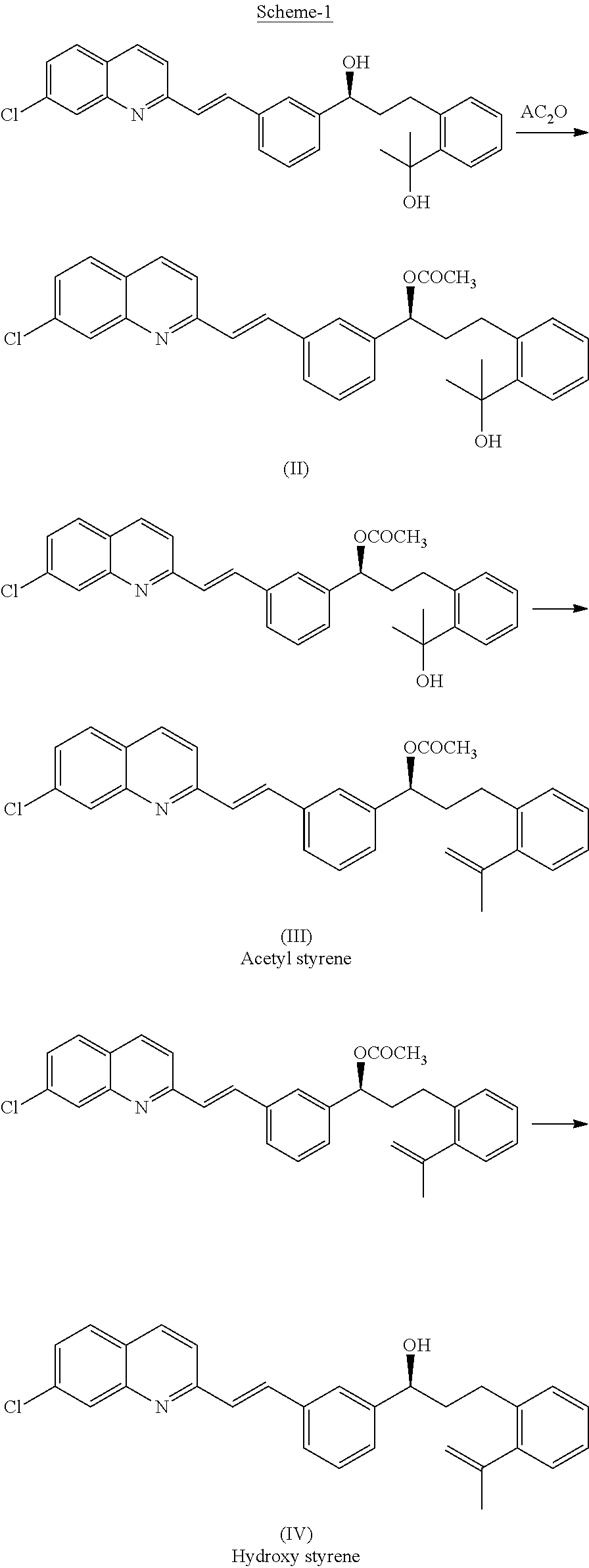
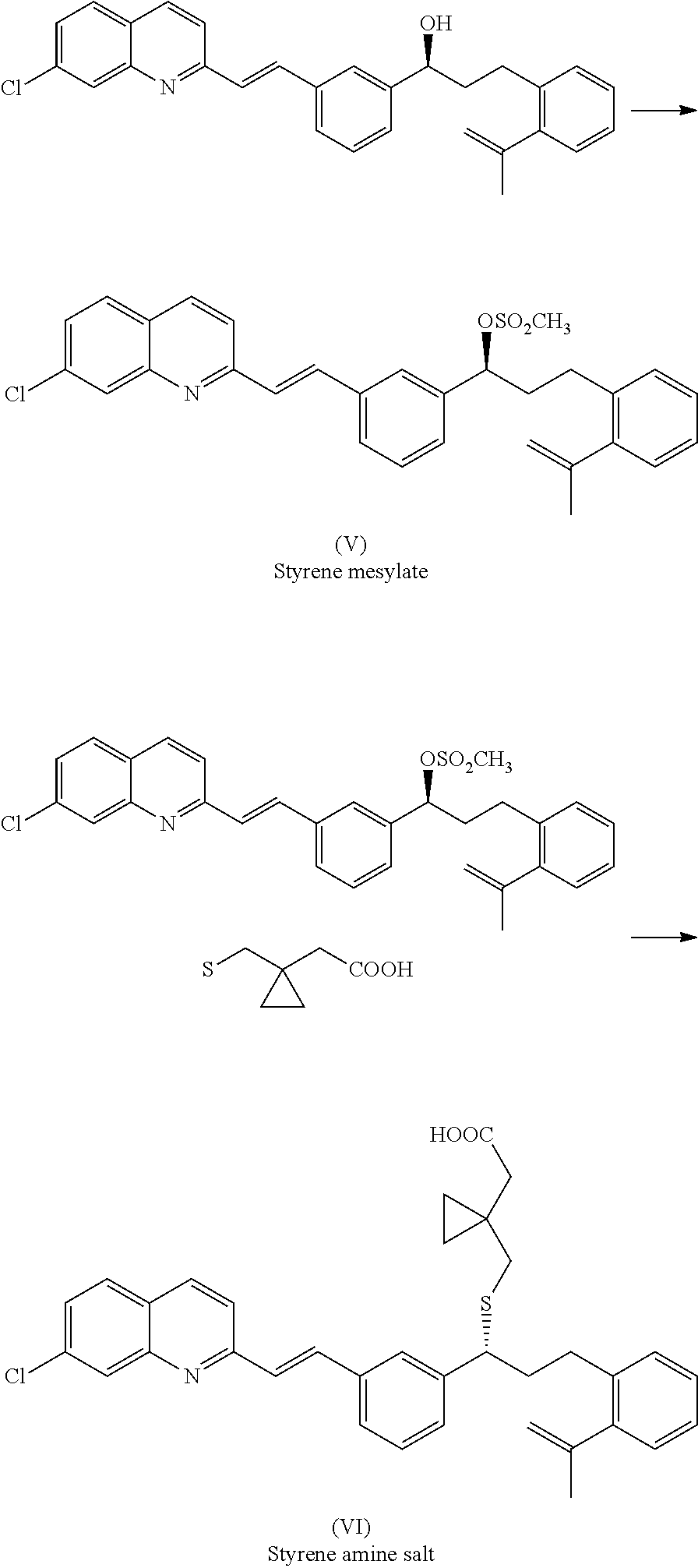
Improved process for preparing montelukast and salts thereof
ActiveUS20110112300A1Speed up the processScalable and economicalOrganic chemistryFree acidCyclopropane
A method for the preparation of montelukast and salts thereof has been described. The method comprises of following steps: (a) (S)-1-(3-((E)-2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-isopropenylphenyljpropyl methane sulphonate (styrene mesylate salt) (b) coupling with 1-(mercapto methyl)cyclopropane acetic acid followed by salification with an amine gives styrene amine salt (c) Converting styrene amine salt to Montelukast amine salt (d) Converting Montelukast amine salt to Montelukast free acid and or its required alkali / alkaline salt.
Owner:LAURUS LABS
Quinoline-1, 2, 4-triazine heterozygote as well as preparation method and application thereof
ActiveCN114133377AGood antimalarial effectOrganic chemistryAntiparasitic agentsQuinolineCombinatorial chemistry
The invention relates to the technical field of medicinal chemistry, in particular to a quinoline-1, 2, 4-triazine heterozygote as well as a preparation method and application thereof. The structure of the chloro-quinoline and 1, 2, 4-triazine heterozygote is as shown in a formula I in the specification. The preparation method of the quinoline-1, 2, 4-triazine heterozygote comprises the following step of: reacting a compound shown as a formula II with a compound shown as a formula III in the presence of sodium carbonate or cesium carbonate to obtain the quinoline-1, 2, 4-triazine heterozygote. The quinoline-1, 2, 4-triazine heterozygote disclosed by the invention has very good antimalarial activity.
Owner:JIANGSU OCEAN UNIV
A kind of preparation method of 4,7-dichloroquinoline
The invention relates to a preparation method of 4,7-dichloroquinoline in the technical field of medicine, which uses 2-amino-6-chlorobenzoic acid (I) as a starting material, and ethoxymethine in an organic solvent Diethyl malonate (II) undergoes condensation reaction to obtain (Ⅲ), III undergoes cyclization, hydrolysis, and acidification to obtain hydroxyquinoline dicarboxylic acid (V), V undergoes high-temperature decarboxylation to obtain hydroxyquinoline (VI), and VI undergoes Chlorination of phosphorus oxychloride gives 4,7-dichloroquinoline. The 4,7-dichloroquinoline prepared by the method of the present invention does not have the isomer 4,5-dichloroquinoline, and the temperature of the condensation reaction under the catalysis of the catalyst is low, which improves the yield of the reaction and avoids the generation of impurities , and reduce energy consumption, improve the economic benefits of the product.
Owner:JIANGSU TIANHE PHARMA CO LTD
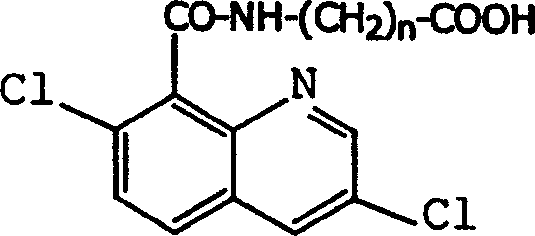
Production method and use for dichloro quinolinic acid artificial hapten, artificial antigen and specific antibody
InactiveCN1240685CEasy to handleFast and accurate analysis and detectionImmunoglobulinsTesting foodAntiendomysial antibodiesSpecific antibody
The invention discloses a process for preparing Quinclorac artificial semiantigen, artificial antigen, specific antibody and use thereof, wherein the preparation comprises, subjecting the dichloroquine (3,7-dichlorine-8-quinoline carboxylic acid) to sulfoxide chlorinated acylation, reacting with reanal and aminocaproic acid, thus obtaining semiantigen 4-(3,7-dichlorine-8-quinolineformyl) reanal or 6-(3,7-dichlorine-8-quinolineformyl) aminocaproic acid (QB or QC).
Owner:ZHEJIANG UNIV
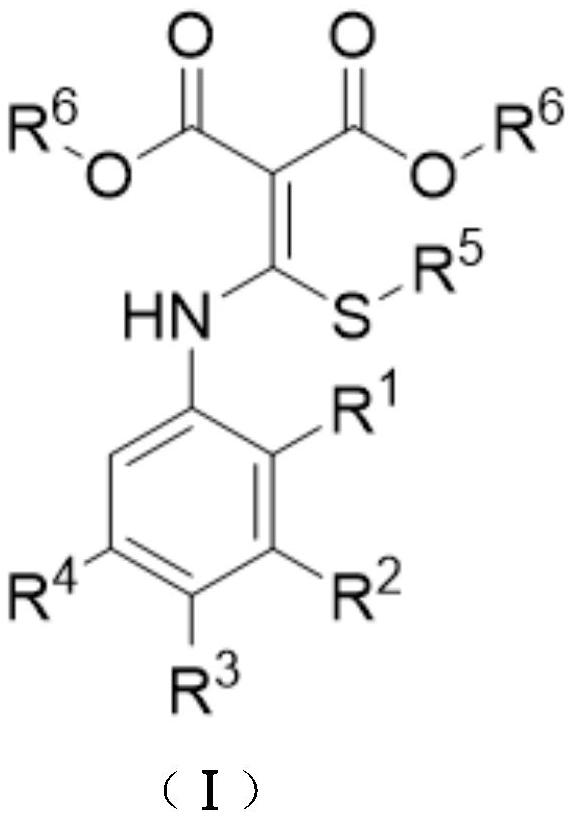
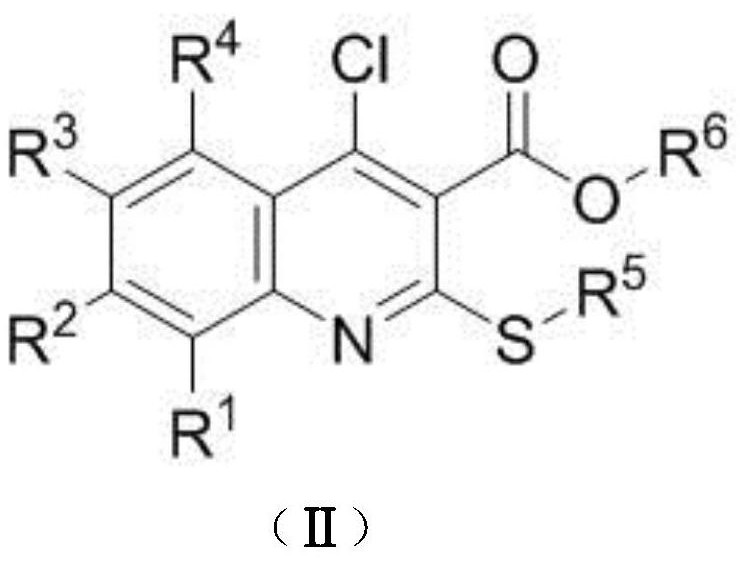
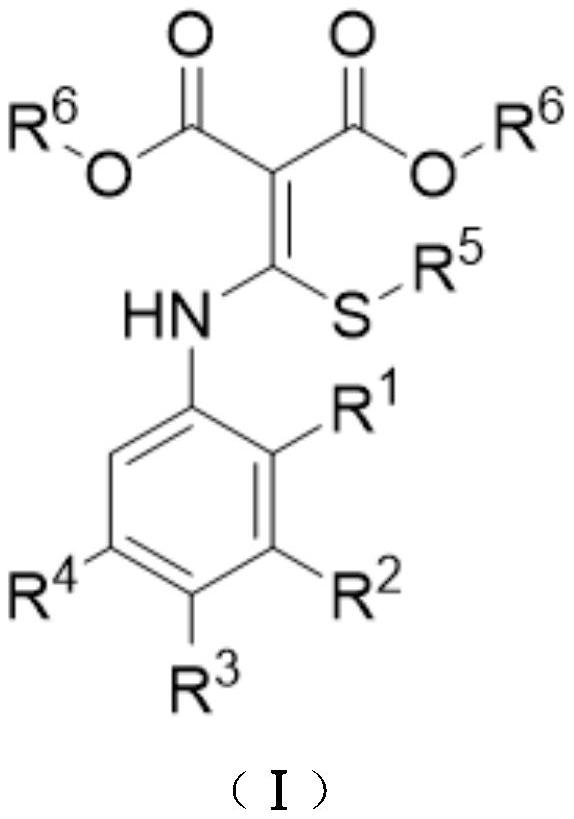
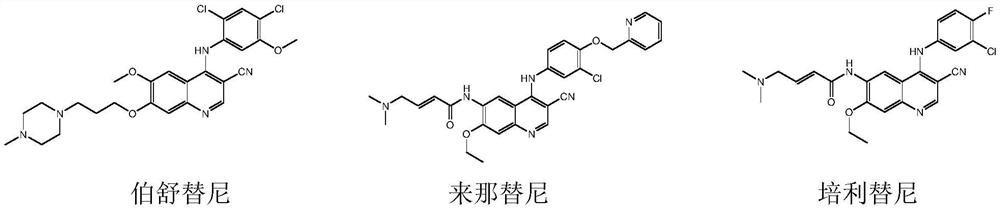
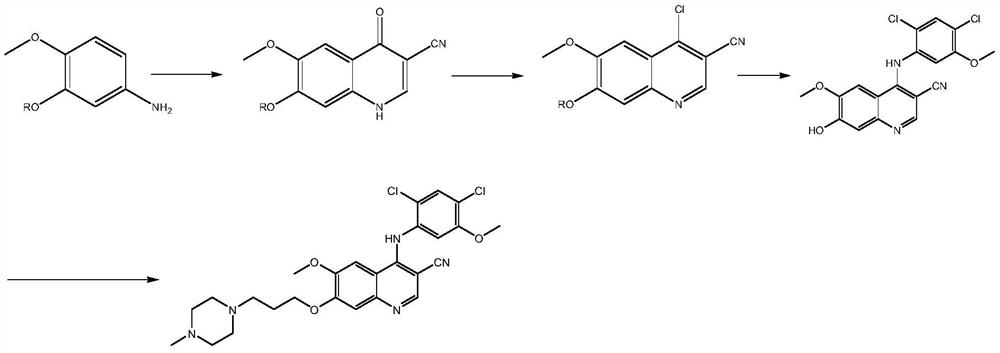
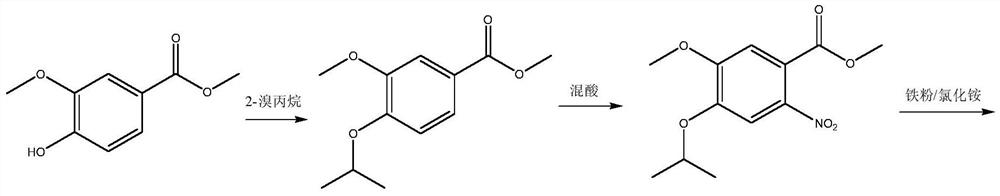
A kind of preparation method of disubstituted 4-chloroquinoline-3-carbonitrile derivative and bosutinib
The invention provides a preparation method of a disubstituted 4-chloroquinoline-3-carbonitrile derivative and a preparation method of bosutinib. The preparation method of the disubstituted 4-chloroquinoline-3-carbonitrile derivative comprises the following steps: disubstituted o-nitrobenzoate (II) used as a raw material and acetonitrile are condensed under the action of an alkali to obtain a compound of formula III; the compound of formula III and a chloroformylating reagent undergo a chloroformylating reaction to obtain a compound of formula IV1 or formula IV2; and the compound of formula IV1 undergoes catalytic hydrogenation cyclization in the presence of a hydrogenation catalyst to prepare 7-[3-(4-methyl-1-piperazinyl)propoxy]-6-methoxy-4-chloroquinoline-3-carbonitrile (Ia), or the compound of formula IV2 is subjected to catalytic hydrogenation cyclization and anhydride amidation to prepare 6-acetamido-7-ethoxy-4-chloroquinolin-3-carbonitrile (Ib). The compound of formula Ia or Ibis used to prepare bosutinib, neratinib or pelitinib. The method of the invention has the advantages of short process flow, simplicity in operation, easiness in realization, low cost, few three wastes, high yield, high purity, and easiness in industrial production.
Owner:XINFA PHARMA
The preparation method of 7-chloro-8-quinoline carboxylic acid
ActiveCN111377863BAvoid it happening againReduce the cost of waste treatmentOrganic chemistryPtru catalystQuinoline
The present invention relates to the field of herbicides, in particular to a preparation method of 7-chloro-8-quinoline carboxylic acid, the method comprising the following steps: 1) dissolving N-hydroxyphthalimides as a catalyst In the reaction liquid of compound and azobisisobutyronitrile, with oxygen as oxidant, 7-chloro-8-methylquinoline is oxidized to obtain 7-chloro-8-quinoline carboxylic acid; 2) the oxidation of step 1) The product is separated into solid and liquid to obtain a solid phase and a reaction liquid phase. Through the method of the invention, the production of a large amount of waste acid and waste water in the synthesis process can be avoided, and the reaction liquid phase can be recovered and used mechanically.
Owner:NUTRICHEM LAB CO LTD
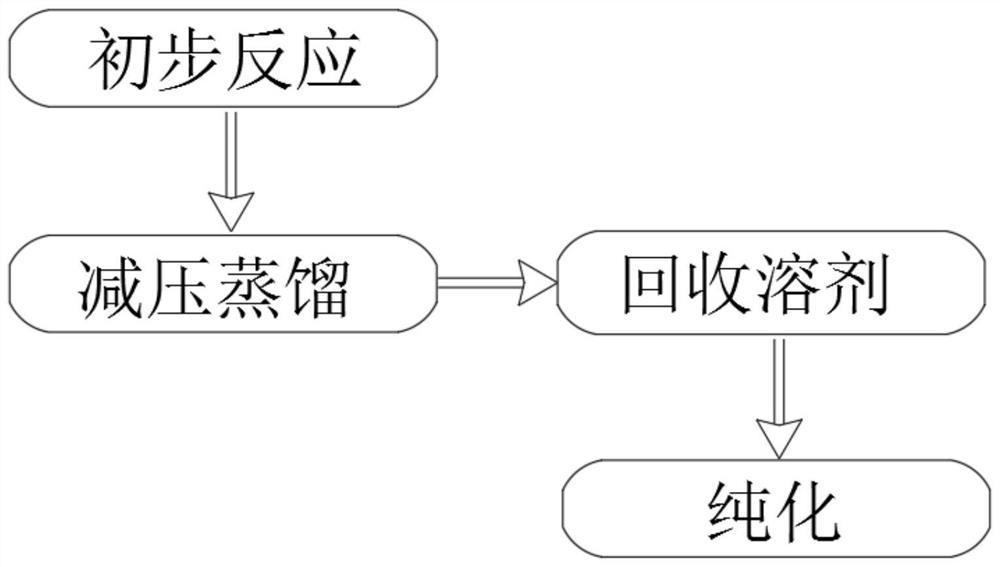
Synthesis and purification method of 4, 7-dichloroquinoline
The present invention relates to the technical field of compound synthesis, and discloses a 4, 7-dichloroquinoline synthesis purification method, which comprises: S1, primary reaction: adding 7-chloro-4-hydroxyquinoline into 300 g of toluene, sequentially adding DMF and triphenylphosphine oxide, starting stirring, internally heating to a temperature of 79-90 DEG C, slowly adding phosphorus oxychloride or a toluene solution of phosphorus oxychloride in a dropwise manner, after the dropwise adding is completed, carrying out a reaction for 2-3 h, and cooling to a room temperature to obtain the 4, 7-dichloroquinoline. Continuously reacting until the TLC monitoring reaction is finished; s2, performing reduced pressure distillation, namely performing reduced pressure distillation on the reaction liquid in the S1. The method has the advantages of simple operation, high yield, no use of any reagent in the purification process, no introduction of new impurities, no special requirements on equipment, no environmental pollution, no introduction of an organic solvent, no pollution, and easy industrial production.
Owner:南京固与生物有限公司
Benzo[e][1,2,4]triazine-1-oxo derivatives, compositions and applications thereof
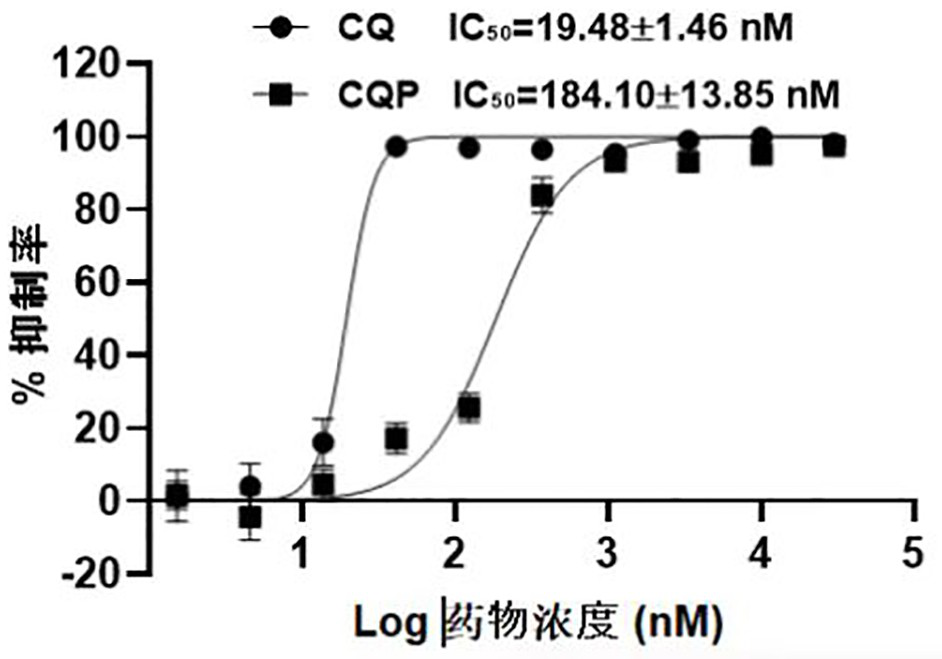
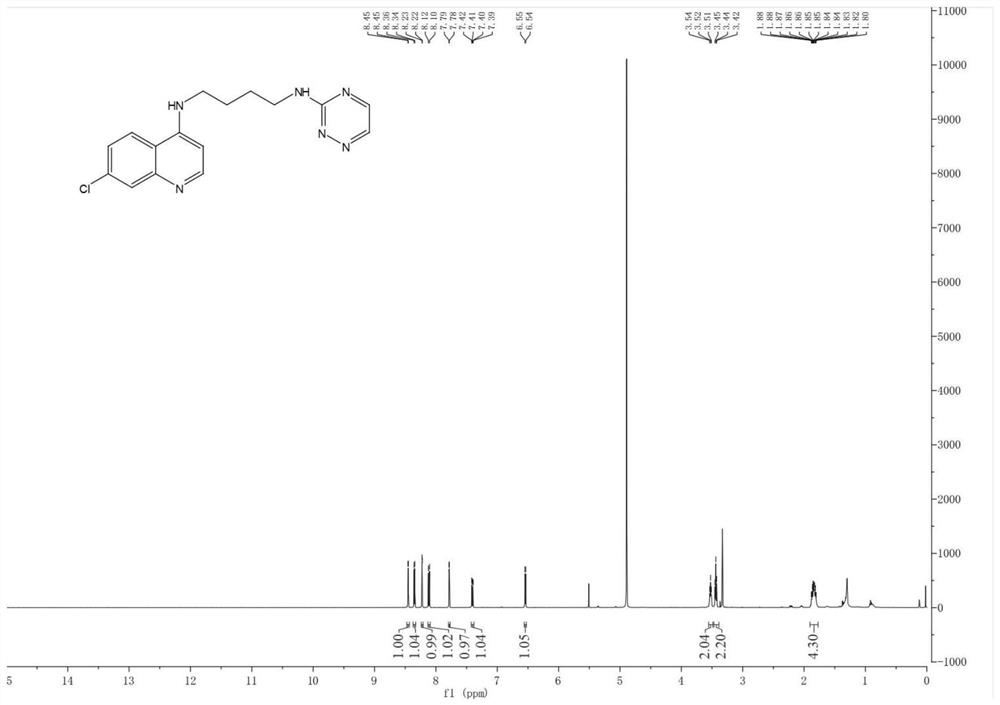
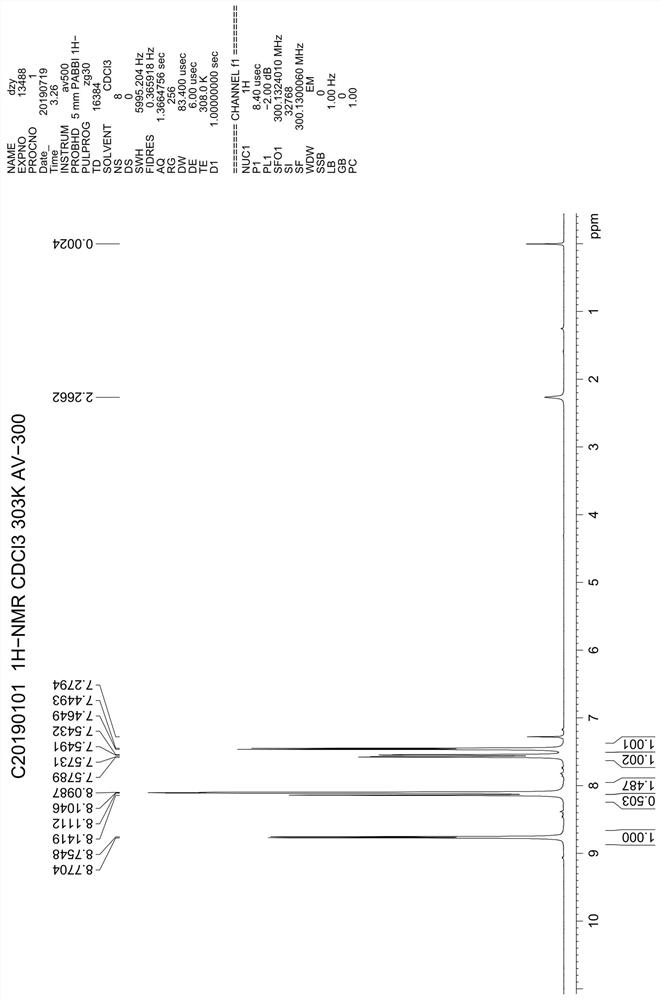
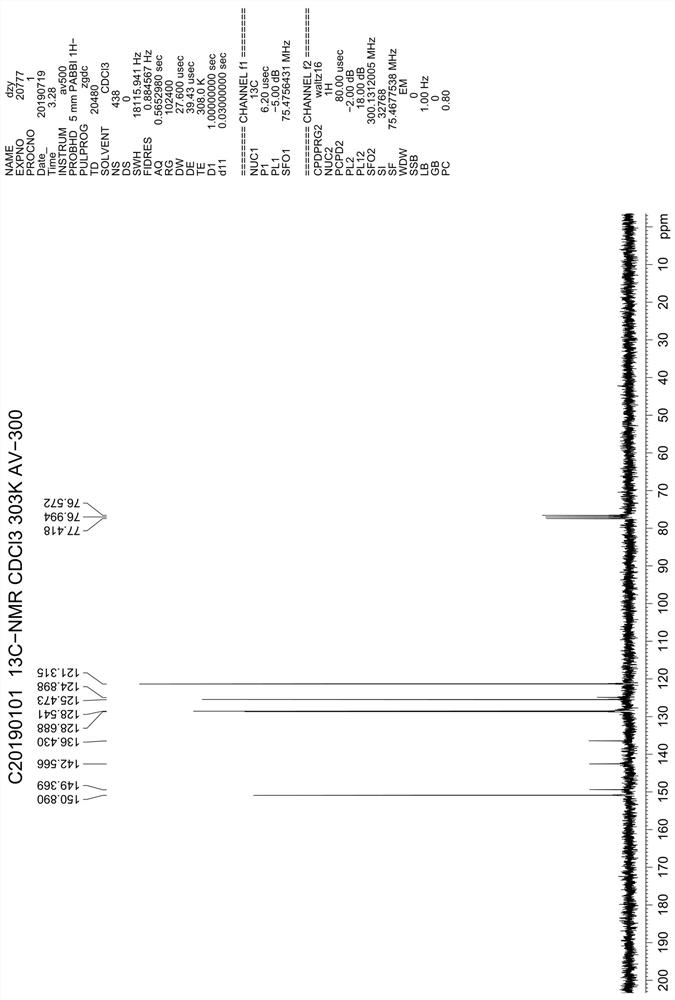
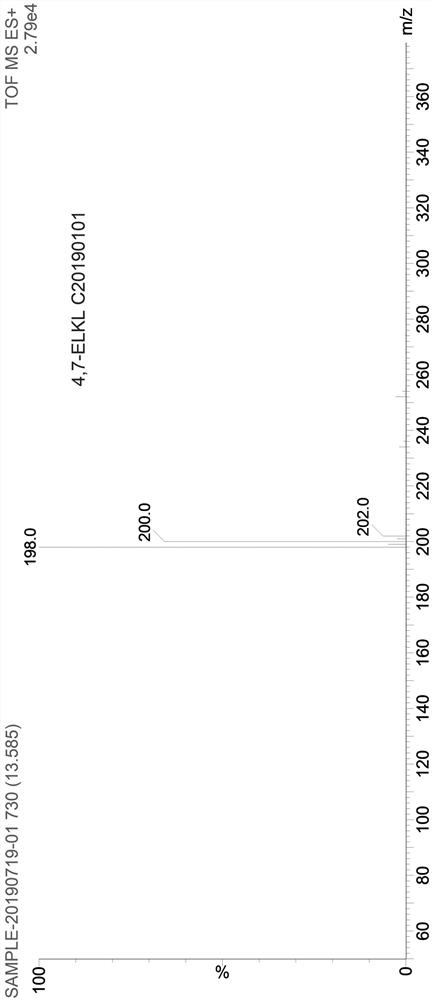
ActiveCN106831711BEfficient killingStrong cytotoxicityOrganic chemistryAntineoplastic agentsBromineQuinoline
The present invention relates to a kind of 3-[(7-chloroquinoline-4-amino)butylamino]-7-substituted-benzo[e][1,2,4]triazine- 1‑Oxygen derivatives, their pharmaceutically acceptable salts, esters, solvates: R 1 selected from hydrogen, hydroxyl, C 1 -C 6 Alkyl, fluorine, chlorine and bromine; R 2 selected from hydrogen, C 1 -C 12 Alkyl or hydroxyl; R 3 selected from hydrogen, C 1 -C 12 Alkyl or hydroxyl. The present invention also provides a composition comprising 3-[(7-chloroquinoline-4-amino)butylamino]-7-substituted-benzo[e][1,2, 4] Triazine‑1‑oxygen derivatives, their pharmaceutically acceptable salts, esters, solvates and pharmaceutically acceptable excipients: wherein, R 1 selected from hydrogen, hydroxyl, C 1 -C 6 Alkyl, fluorine, chlorine or bromine; R 2 selected from hydrogen, C 1 -C 12 Alkyl or hydroxyl; R 3 selected from hydrogen, C 1 -C 12 Alkyl or hydroxyl. The present invention also claims protection of 3-[(7-chloroquinoline-4-amino)butylamino]-7-substituted-benzo[e][1,2,4]triazine-1-oxygen derivatives, and its pharmaceutical The application of acceptable salts, esters and solvates in the preparation of tumor therapeutic drugs.
Owner:SUZHOU UNIV
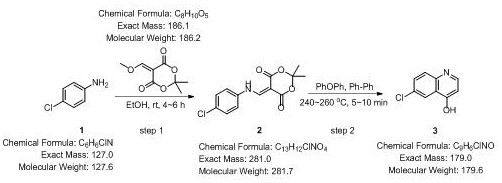
Novel process for synthesizing 4-hydroxy-6-chloroquinoline
The invention belongs to the technical field of synthesis of 4-hydroxy-6-chloroquinoline, and particularly relates to a novel process for synthesizing 4-hydroxy-6-chloroquinoline. The process comprises the following steps: S1, cleaning a first reaction container, drying and cooling the container to room temperature, adding parachloroaniline and 5-(methoxymethylene)-2, 2-dimethyl-1, 3-dioxo-4, 6-diketone into an ethanol solution with a certain concentration according to a molar ratio of (0.1-1):(0.1-1), performing slow stirring, and performing reacting for 4-6 hours until the reaction is finished; S2, carrying out suction filtration on the reaction product to obtain 80-95% A; S3, adding a certain amount of diphenyl ether-biphenyl eutectic into a second reaction container, heating the container to 240-260 DEG C, adding the product A, performing stirring,performing reacting for 5-10 minutes, and performing cooling to room temperature; and S4, carrying out suction filtration on a product obtained in the S3 in petroleum ether to obtain 80-90% 4-hydroxy-6-chloroquinoline, and performing drying to obtain a 4-hydroxy-6-chloroquinoline product. According to the method disclosed by the invention, spin-drying is changed into suction filtration, so that a solid product with very high purity can be obtained, and the time is also saved; and in addition, the diphenyl ether-biphenyl eutectic is used as the solvent, so that time, manpower and material resources can be saved.
Owner:DEZHOU UNIV
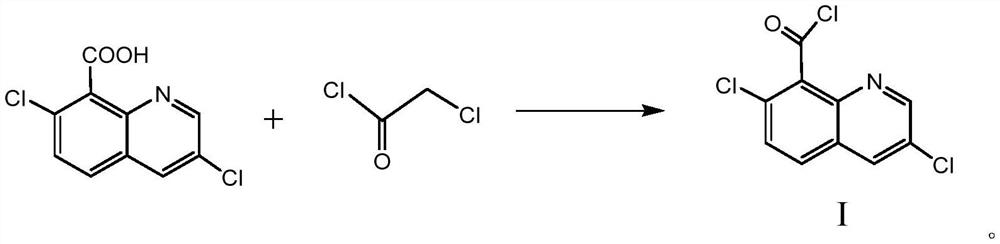
A kind of synthetic method of 3,7-dichloro-8-quinoline formyl chloride
The invention relates to a method for synthesizing 3,7-dichloro-8-quinolineformyl chloride. Under the condition that no solvent exists, 3,7-dichloro-8-quinolinic acid, monochloroacetyl chloride and a catalyst The acylation reaction is carried out at 95°C-120°C, after filtration, washing and distillation under reduced pressure, the compound represented by formula I is obtained, and the specific synthetic route is as follows. In the process of the reaction, a catalyst is used, no solvent needs to be added, the source of raw materials is easily available, the cost is low, the process steps are few, the reaction conditions are mild, no special equipment is required, the product quality is good, the purity is high, and the yield is higher than 95%, No waste water and waste gas, relatively safe and environmentally friendly.
Owner:赵东源 +1
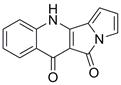
A kind of method for preparing peninoan compound
ActiveCN108623581BOrganic chemistryMetabolism disorderOrganic synthesisTert-Butyloxycarbonyl protecting group
The invention belongs to the field of organic synthesis, and in particular relates to a synthesis method of penicinotam compounds. The present invention uses aniline and ethoxymethylene malonate as starting materials through condensation reaction, Gould-Jacob reaction to form quinoline parent ring, and then through bromination and Suzuki reaction to obtain 2-(1-(tert Butoxycarbonyl)‑1 H -pyrrole-2-yl)-4-chloroquinoline-3-formic acid ethyl ester intermediate, and finally prepare the target product peninoan through ring-closing reaction.
Owner:OCEAN UNIV OF CHINA
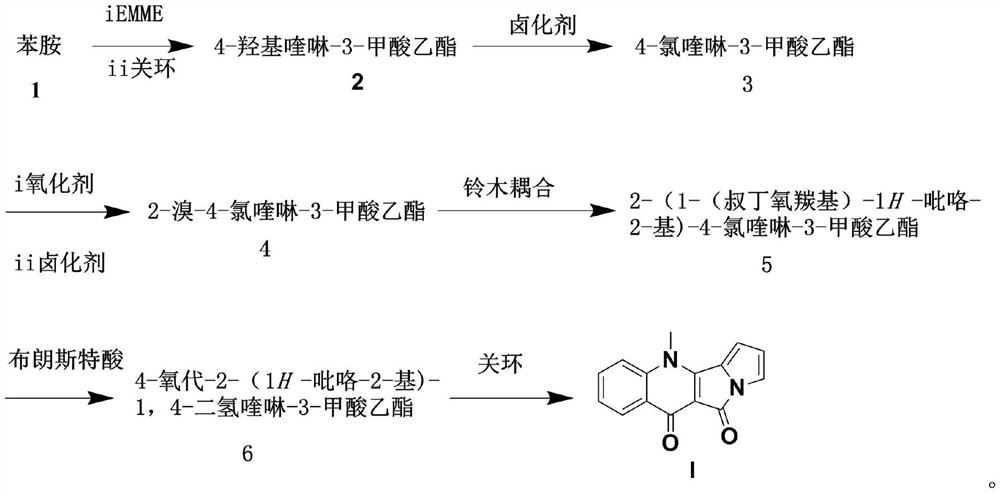
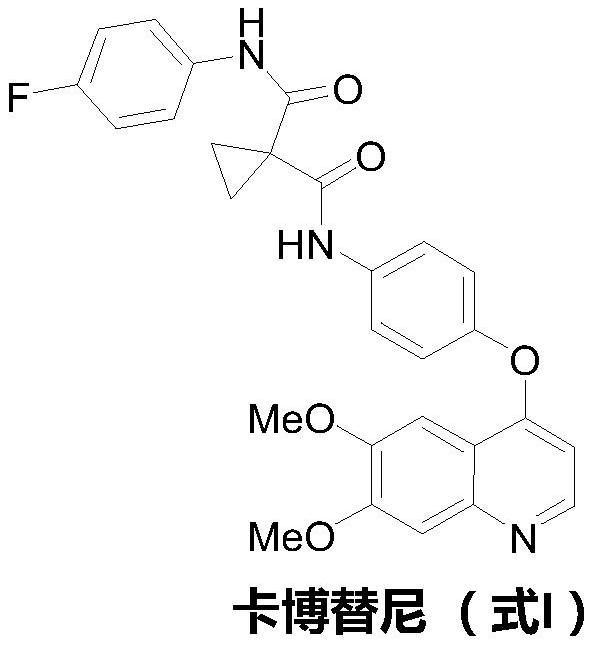
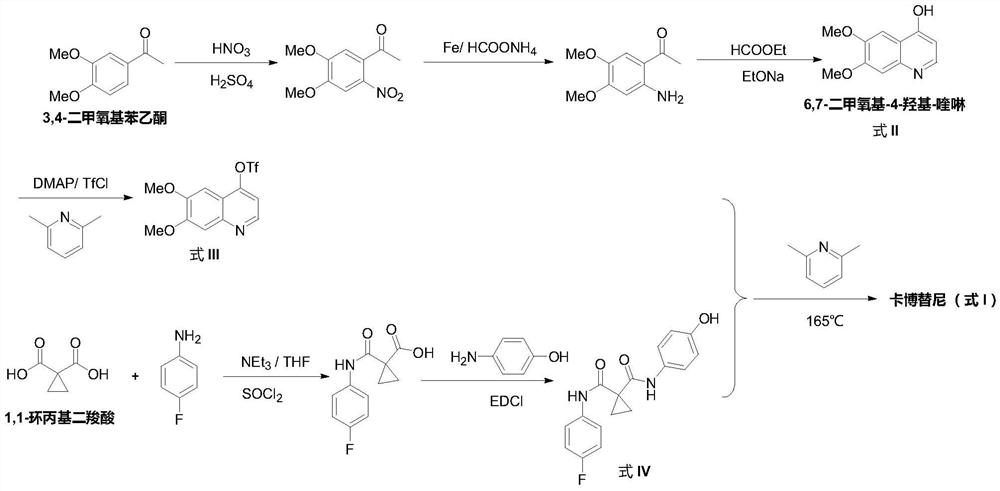
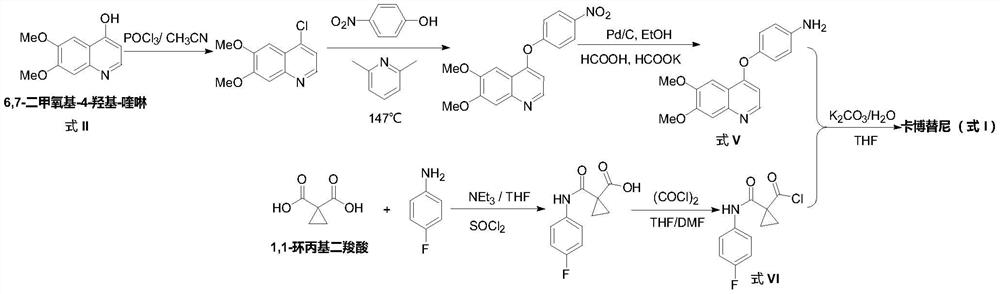
The preparation method of cabozantinib
ActiveCN110117254BEmission reductionImprove reaction efficiencyOrganic chemistryCarboxyl radicalBiochemical engineering
The object of the present invention is to provide a more efficient method for synthesizing cabozantinib, which uses 6-nitroveratraldehyde as a starting material, and completes reduction, cyclization, and hydrolysis reactions in one pot to obtain 6,7- Dimethoxy-2-carboxy-quinoline, then treated with thionyl chloride to obtain 6,7-dimethoxy-2-carboxy-4-chloro-quinoline, and finally again under copper catalyst system conditions The kettle method completes the condensation and decarboxylation reactions to obtain the target product cabozantinib.
Owner:江苏君若药业有限公司 +2
Synthesis method of 4-chloroquinoline compound
The invention discloses a synthesis method of a 4-chloroquinoline compound, and belongs to the technical field of organic synthesis. The method comprises the following steps: dissolving triphosgene in an organic solvent at room temperature, adding an N,S-ketene acetal compound, sealing a reaction system, conducting heating to 90-140 DEG C, continuing stirring for reaction for 2-5 hours, and post-treating the obtained reaction liquid to obtain the 4-chloroquinoline compound. The method for synthesizing the 4-chloroquinoline compound has the advantages of few steps, high yield and safe and convenient operation.
Owner:LIAONING UNIVERSITY
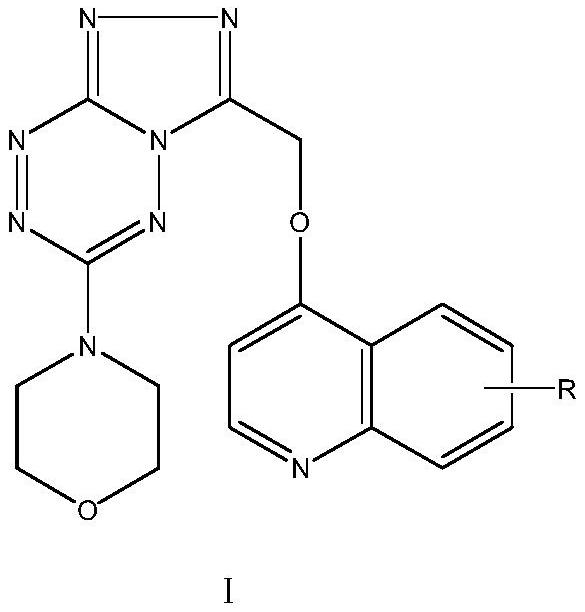
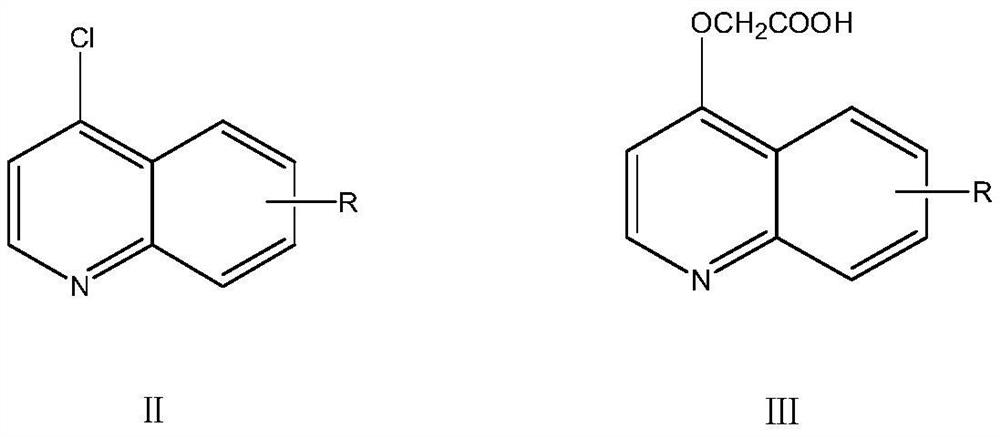
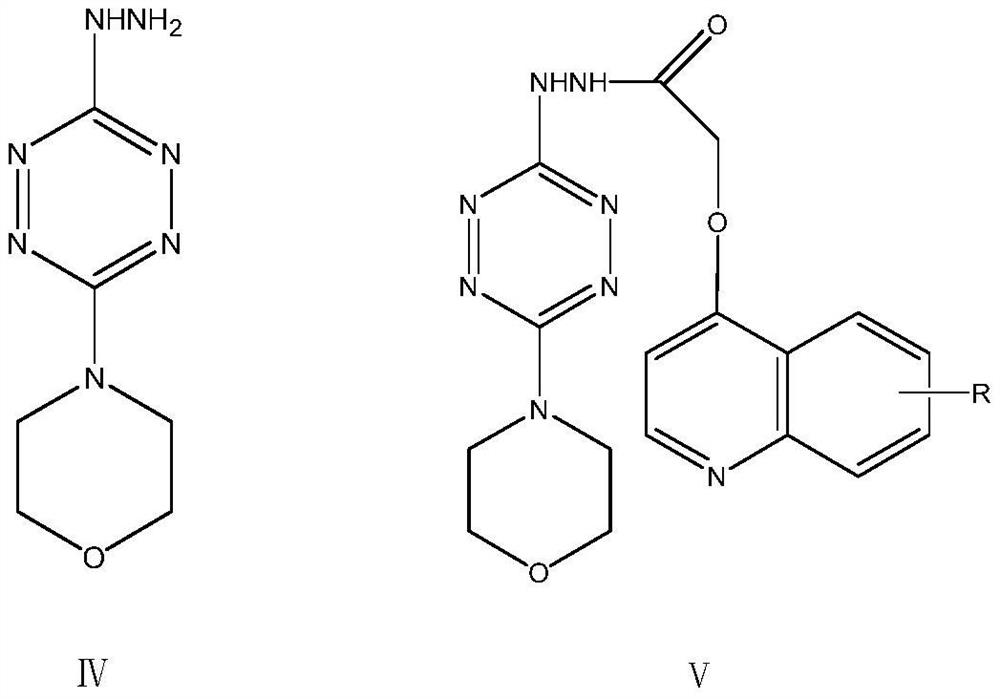
Triazolotetrazine compound containing morpholine and quinoline rings as well as preparation method and application thereof
ActiveCN113788835AStrong in vitro antitumor activityHigh inhibitory activityOrganic chemistryAntineoplastic agentsMorpholineChemical compound
The invention relates to a triazolotetrazine compound containing morpholine and quinoline rings as well as a preparation method and application thereof, and belongs to the technical field of medicine synthesis. The invention aims to provide a novel anti-tumor compound, and provides a triazolotetrazine compound containing morpholine and quinoline rings as well as a preparation method and application thereof. The preparation method of the triazolotetrazine compound comprises the steps that inorganic strong base, glycollic acid and 4-chloroquinoline are subjected to a reaction, and after the reaction is finished, the reaction system is adjusted to be acidic; and a reaction is carried out on an intermediate and 4-(6-hydrazino-1, 2, 4, 5-tetrazine-3-yl) morpholine under the action of a condensing agent, and then a cyclization reaction is carried out under the action of phosphorus oxychloride to obtain the triazolo tetrazine compound containing morpholine and quinoline rings, wherein the triazolo tetrazine compound can be used for preparing drugs or functional foods for treating and resisting tumors. The compound has the advantages of inhibition activity on c-Met kinase, in-vitro anti-tumor activity, short reaction route and easiness in operation.
Owner:TAIZHOU VOCATIONAL & TECHN COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Chromeno[3',4':5,6]pyrano[2,3-b]quinoline derivative, preparation method and application thereof Chromeno[3',4':5,6]pyrano[2,3-b]quinoline derivative, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/5e6c549e-ae0a-49a6-a97f-c58e824c3186/BDA0001298198750000021.png)
![Chromeno[3',4':5,6]pyrano[2,3-b]quinoline derivative, preparation method and application thereof Chromeno[3',4':5,6]pyrano[2,3-b]quinoline derivative, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/5e6c549e-ae0a-49a6-a97f-c58e824c3186/BDA0001298198750000031.png)
![Chromeno[3',4':5,6]pyrano[2,3-b]quinoline derivative, preparation method and application thereof Chromeno[3',4':5,6]pyrano[2,3-b]quinoline derivative, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/5e6c549e-ae0a-49a6-a97f-c58e824c3186/BDA0001298198750000041.png)
![Benzo[e][1,2,4]triazine-1-oxo derivatives, compositions and applications thereof Benzo[e][1,2,4]triazine-1-oxo derivatives, compositions and applications thereof](https://images-eureka.patsnap.com/patent_img/8651e41b-1a6e-4300-9541-a491094197bb/HDA0001199572970000011.png)
![Benzo[e][1,2,4]triazine-1-oxo derivatives, compositions and applications thereof Benzo[e][1,2,4]triazine-1-oxo derivatives, compositions and applications thereof](https://images-eureka.patsnap.com/patent_img/8651e41b-1a6e-4300-9541-a491094197bb/HDA0001199572970000012.png)
![Benzo[e][1,2,4]triazine-1-oxo derivatives, compositions and applications thereof Benzo[e][1,2,4]triazine-1-oxo derivatives, compositions and applications thereof](https://images-eureka.patsnap.com/patent_img/8651e41b-1a6e-4300-9541-a491094197bb/HDA0001199572970000013.png)