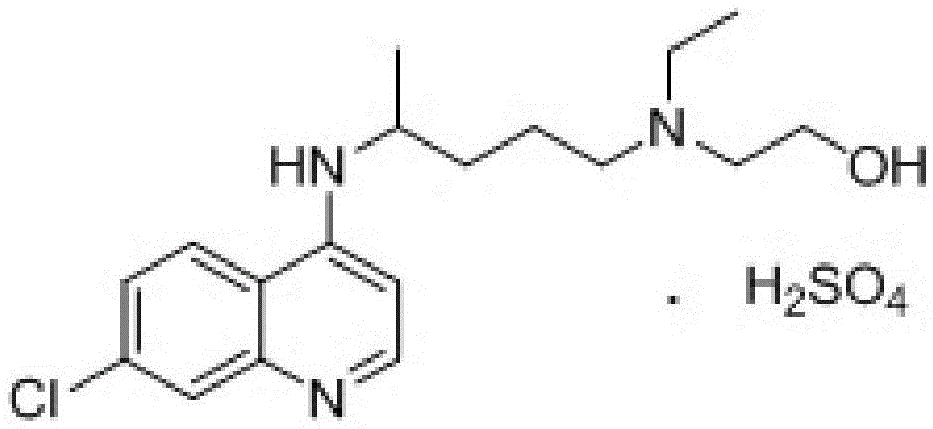
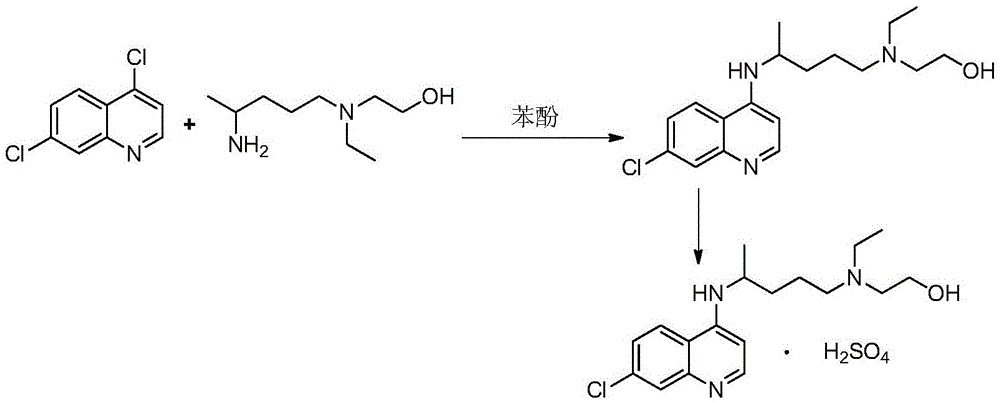
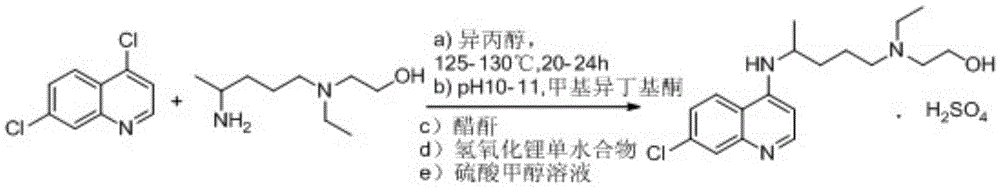
A kind of industrialized preparation method of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and hydroxychloroquine sulfate, which is applied in the field of preparation of hydroxychloroquine sulfate, can solve problems such as unfavorable recycling and environmental protection, increase production costs, and cumbersome production processes, so as to save extraction agent and labor costs, and save production Cost, the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of hydroxychloroquine
[0034] Add 20g of side chains and 22.4g of 4.7-dichloroquinoline into a three-necked flask, protect it with argon, raise the temperature to 100°C, stir for 1h, then raise the temperature to 120-130°C for 20h. After the reaction is complete, lower the temperature slightly, add 40g of water to the reaction solution, then add 20g of concentrated sulfuric acid, add 80g of liquid caustic soda after stirring, stir for 30min to separate the liquid, discard the water phase; add 60g of ethyl acetate to the organic phase, and wait for the organic phase to The phase was dissolved, then cooled to 0-10°C, kept warm for 2h, filtered with suction, and dried to obtain 34g of crude hydroxychloroquine, with a yield of 89% and HPLC ≥ 95%.
Embodiment 2
[0035] Embodiment 2: the preparation of hydroxychloroquine
[0036] Add 20g of side chains and 22.4g of 4.7-dichloroquinoline into a three-necked flask, protect it with nitrogen, raise the temperature to 100°C, stir for 1h, then raise the temperature to 120-130°C for 20h. After completion of the reaction, lower the temperature slightly, add 20 g of water to the reaction solution, then add 40 g of concentrated hydrochloric acid, add 80 g of liquid caustic soda after stirring, stir for 30 min to separate the liquid, discard the water phase; add 60 g of ethyl acetate to the organic phase, and wait for the organic phase to phase was dissolved, then cooled to 0-10°C, kept warm for 2h, filtered with suction, and dried to obtain 33.6g of crude hydroxychloroquine, with a yield of 88%, HPLC≥96%
Embodiment 3
[0037] Embodiment 3: the preparation of hydroxychloroquine
[0038] Add 20g of side chains and 22.4g of 4.7-dichloroquinoline into a three-necked flask, protect it with carbon dioxide, raise the temperature to 100°C, stir for 1h, then raise the temperature to 120-130°C for 20h. After the reaction is complete, lower the temperature slightly, add 20g of water to the reaction solution, then add 40g of concentrated hydrochloric acid, add 80g of liquid caustic soda after stirring, stir for 30min to separate the liquid, discard the water phase; add 60g of 1.2-dichloroethane to the organic phase Alkanes, to be dissolved in the organic phase, then lower the temperature to 0 ~ 10 ° C, heat preservation 2h, suction filtration, drying, to obtain crude hydroxychloroquine 33.8g, yield 89%, HPLC ≥ 96%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com