Novel process for synthesizing 4-hydroxy-6-chloroquinoline
A chloroquinoline and new process technology, which is applied in the field of 4-hydroxy-6 chloroquinoline synthesis new process, can solve the problems of long reaction time, waste of time and labor, low product purity, etc., and achieves the effect of saving labor and time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] A specific embodiment of the present invention will be described in detail below, but it should be understood that the protection scope of the present invention is not limited by the specific embodiment.
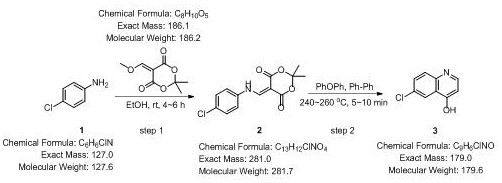
[0017] refer to figure 1 Shown, a kind of 4-hydroxyl-6 chloroquinoline synthetic new technique comprises the following steps:
[0018] S1. Clean the first reaction container, dry it, cool it to room temperature, and mix p-chloroaniline and 5-(methoxymethylene)-2,2-dimethyl-1,3-dioxo-4,6-diketone Put it into a certain concentration of ethanol solution at a molar ratio of 0.1-1:0.1-1, stir slowly, and react for 4-6 hours until the reaction is complete. The reaction conditions are normal temperature and pressure, and it is synthesized by a one-pot method;
[0019] S2, the reaction product is suction-filtered to obtain 80%-95% of A, and the method of suction filtration is simple, quick, green and environmentally friendly, and the obtained product has high purity;
[002...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com