Production method and use for dichloro quinolinic acid artificial hapten, artificial antigen and specific antibody
A technology of quinclorac and artificial hapten, which is applied in the direction of chemical instruments and methods, specific peptides, material inspection products, etc., can solve problems such as unsuitable for large-scale sample detection and analysis, easy to explode, and human danger, etc., to achieve convenient On-site monitoring, high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] 3 Preparation of antibody and establishment of quinclorac ELISA method
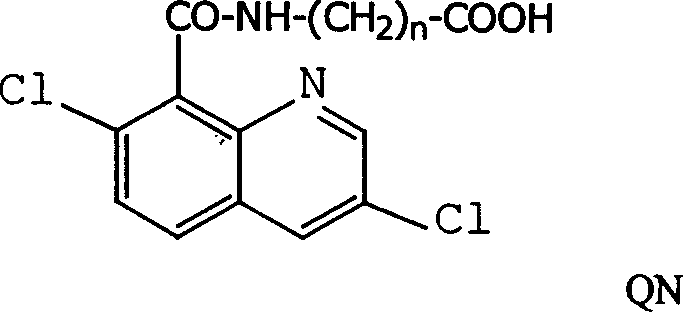
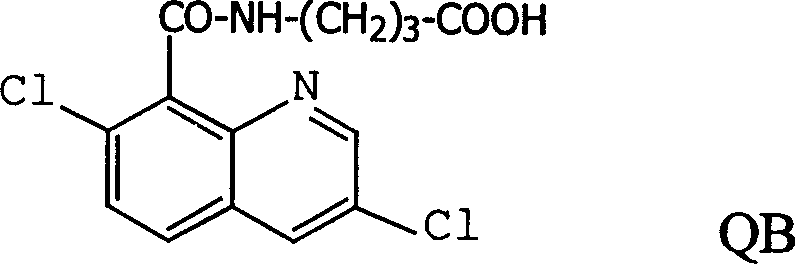
[0042]Three kinds of immunogen complexes (Q-BSA, QC-BSA, QB-BSA) were used to immunize three rabbits according to conventional methods. From the second time of booster immunization, blood was collected from the rabbit's ear vein on the 8th day after each immunization, and the titer of the serum was determined by indirect ELISA after appropriate dilution. By the time of the fourth immunization, the rabbits obtained high-titer antibodies, and the titers after purification and freeze-dried powder were 2000, 1.2×10 6 , 1.6×10 6 . Since the titer of anti-Q-BSA antibody was too low, no further experiments will be performed.
[0043] Using quinclorac antigen antibody immune reaction and enzymatic reaction to establish quinclorac ELISA method. It has high specificity and sensitivity in the detection and analysis of quinclorac residues in food, plants, environmental soil and water samples, the lowest de...
Embodiment 1
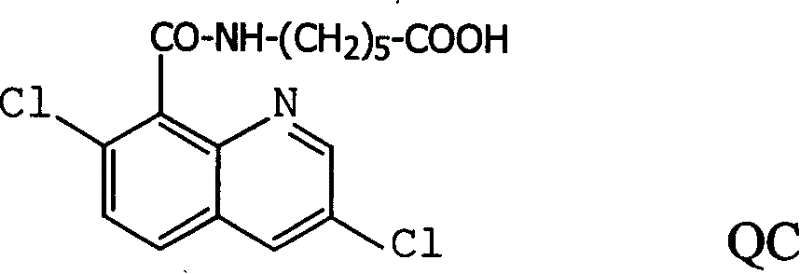
[0044] Example 1: Synthesis of Hapten QC
[0045] 1) Dilute 37.5ml of SOCl in excess of thionyl chloride 2 And 2.42g (10mmol) quinclorac is packed in the there-necked flask, under magnetic stirring, reflux reaction 1.5h, distillation removes excess SOCl 2 , That is, the product 3,7-dichloro-8-quinoline formyl chloride.
[0046] 2) According to the feeding ratio of 6-aminocaproic acid: 3,7-dichloro-8-quinoline formyl chloride = 1:1, put 1.32g (10mmol) of 6-aminocaproic acid into a three-necked flask, and add 5ml of 2mol / L of NaOH solution, stirred to dissolve, 2.60g (10mmol) 3,7-dichloro-8-quinoline formyl chloride was dissolved in 30ml of dioxane, added to the constant pressure dropping funnel, and the solution was added dropwise five times , and then add 2mol / L NaOH solution to keep the pH value of the reaction solution at 8-10. After the addition is completed, continue to react at 0-4°C for 2h. Rise to room temperature, adjust the pH value of the reaction solution to abo...
Embodiment 2
[0049] Example 2: Synthesis of Hapten QC
[0050] 1) Dilute 37.5ml of SOCl in excess of thionyl chloride 2 And 2.42g (10mmol) quinclorac is packed in the there-necked flask, under magnetic stirring, reflux reaction 1.5h, distillation removes excess SOCl 2 , That is, the product 3,7-dichloro-8-quinoline formyl chloride.
[0051] 2) According to the feeding ratio of 6-aminocaproic acid: 3,7-dichloro-8-quinoline formyl chloride = 1.2: 1, put 1.584g (12mmol) of 6-aminocaproic acid into a three-necked flask, add 6ml2mol / L of NaOH solution, stirred and dissolved, 2.60g (10mmol) 3,7-dichloro-8-quinoline formyl chloride was dissolved in 30ml of dioxane, added to the constant pressure dropping funnel, and added dropwise to the solution five times, Then add 2mol / L NaOH solution to keep the pH value of the reaction solution at 8-10. After the addition is complete, continue to react at 0-4°C for 3h. Rise to room temperature, adjust the pH value of the reaction solution to about 4.0 wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com