Quinoline-1, 2, 4-triazine heterozygote as well as preparation method and application thereof
A hybrid, quinoline technology, applied to quinoline-1, can solve problems such as hindering the elimination of malaria, and achieve the effect of good antimalarial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The preparation method of the compound shown in formula II takes following method:
[0063]
[0064] Reaction of 4,7-dichloroquinoline with diamines (C1, C2 and C5): Weigh 4,7-dichloroquinoline (5.05mmol) and dissolve it in 20mL of absolute ethanol, add diamine (15.15mmol) and mix well , heated and stirred at 110°C, refluxed for 12-18 hours, TLC monitored the end of the reaction, stopped the reaction, waited for the reaction system to cool down to room temperature, removed absolute ethanol by rotary evaporation under reduced pressure, added ethyl acetate and saturated saline for extraction, and took the upper organic Anhydrous sodium sulfate was added to the phase to remove water, and then the organic phase liquid was evaporated and dried under reduced pressure with a rotary evaporator to obtain a crude product, which was separated and purified by silica gel column chromatography to obtain the product.
[0065] Reaction of 4,7-dichloroquinoline with alcohol amine (C3...
Embodiment 1
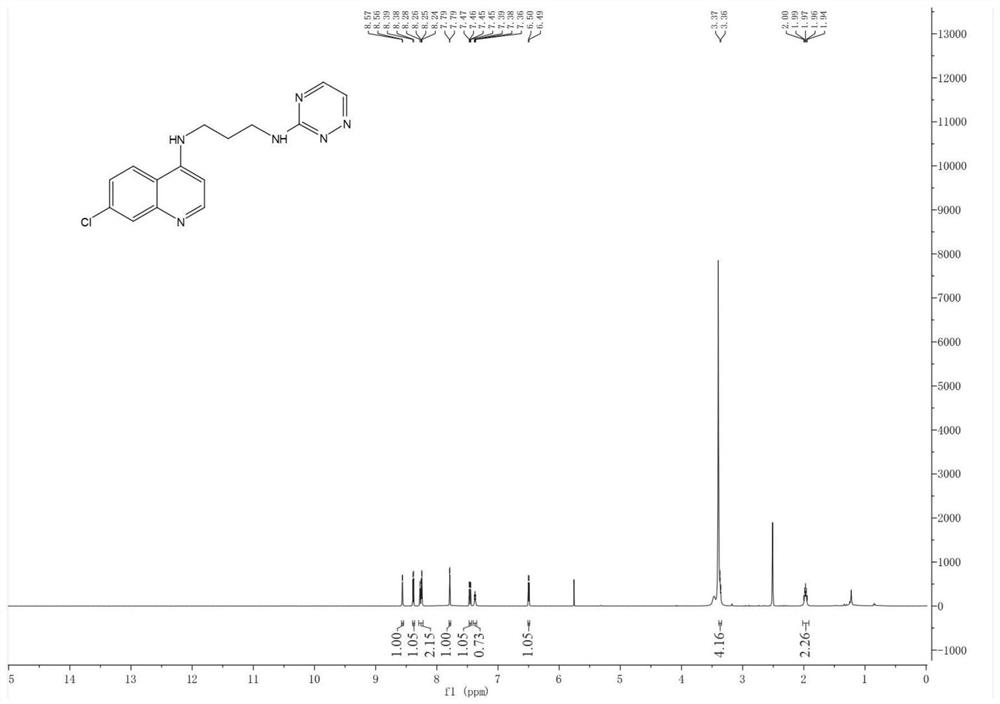
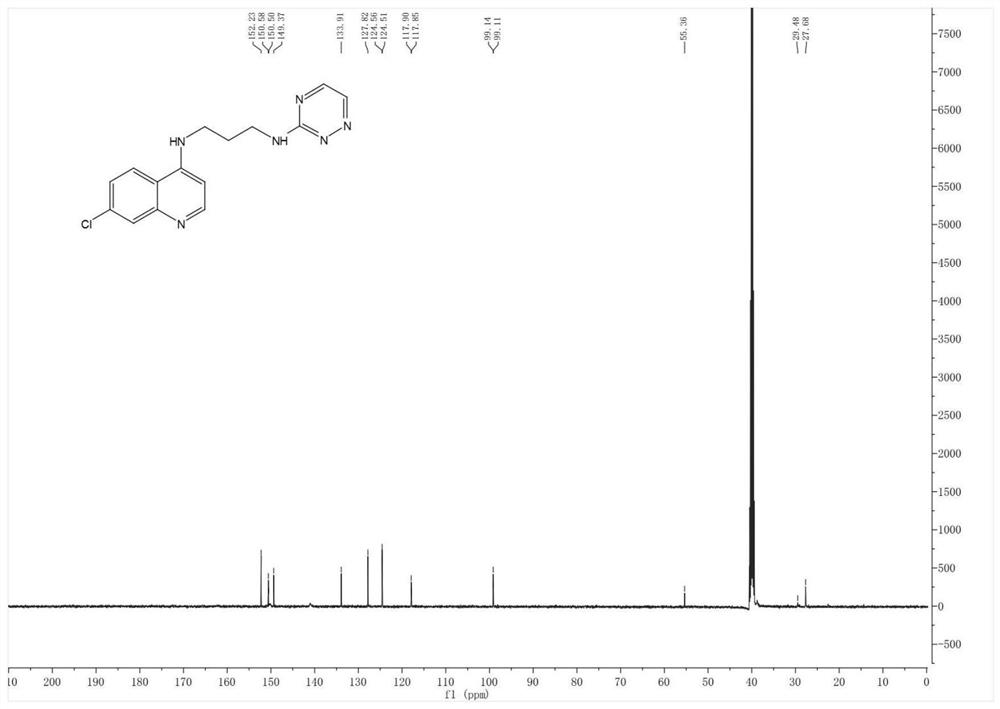
[0072] Embodiment 1: Preparation of N1-(7-chloroquinolin-4-yl)-N3-(1,2,4-triazin-3-yl)propane-1,3-diamine
[0073]
[0074] Take a 50mL flask, accurately weigh 4,7-dichloroquinoline (5.05mmol) into the flask, add 20mL of absolute ethanol and stir until dissolved, add 1,3 propylenediamine (15.15mmol) and mix evenly, heat and stir at 90°C , reflux reaction for 12 hours, TLC monitors the end of the reaction, stop the reaction, wait for the reaction system to drop to room temperature, remove absolute ethanol by rotary evaporation under reduced pressure, add ethyl acetate and saturated brine for extraction, take the upper organic phase and add anhydrous sodium sulfate to dry and remove water, and then use a rotary evaporator to remove ethyl acetate under reduced pressure to obtain a solid crude product, which was separated and purified by silica gel column chromatography (eluent: dichloromethane:methanol=20:1) to obtain intermediate II.
[0075] Take a 250mL flask, weigh 3-methy...
Embodiment 2
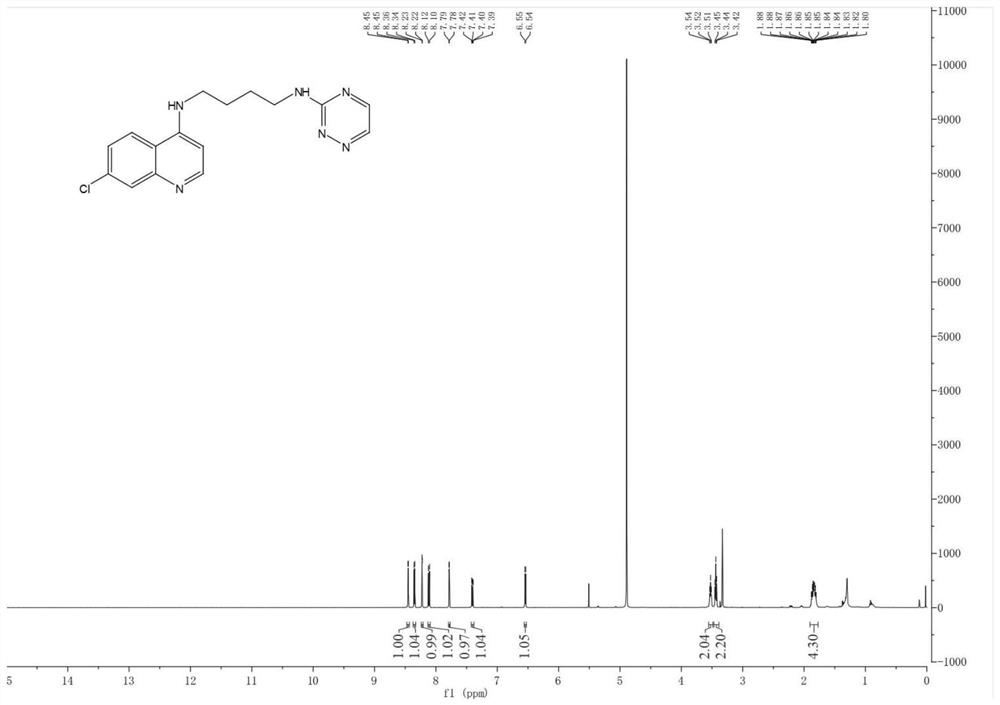
[0077] Example 2: Preparation of N1-(7-chloroquinolin-4-yl)-N4-(1,2,4-triazin-3-yl)butane-1,4-diamine
[0078]
[0079] The preparation method refers to Example 1. A white solid was obtained, yield 50.6%, m.p.157-159°C, 1 H NMR (500MHz, Methanol-d 4 )δ8.45(d, J=2.2Hz, 1H), 8.35(d, J=5.7Hz, 1H), 8.22(d, J=2.2Hz, 1H), 8.11(d, J=9.0Hz, 1H) ,7.78(d,J=2.1Hz,1H),7.40(dd,J=9.0,2.2Hz,1H),6.54(d,J=5.7Hz,1H),3.52(t,J=6.6Hz,2H) ,3.44(t,J=6.7Hz,2H),1.90–1.77(m,4H). 13 C NMR (126MHz, Methanol-d 4 )δ151.43, 150.79, 150.54, 148.05, 139.77, 134.99, 129.44, 129.43, 125.99, 124.58, 122.94, 117.33, 98.25, 42.30, 25.25
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com