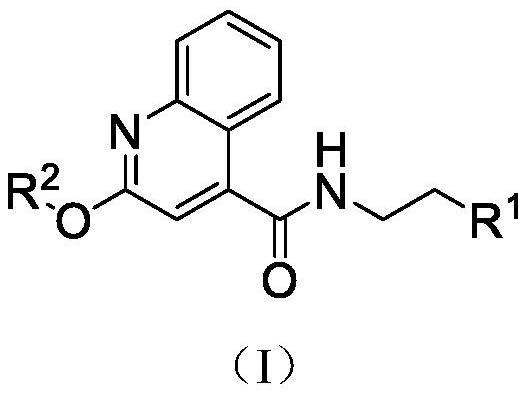
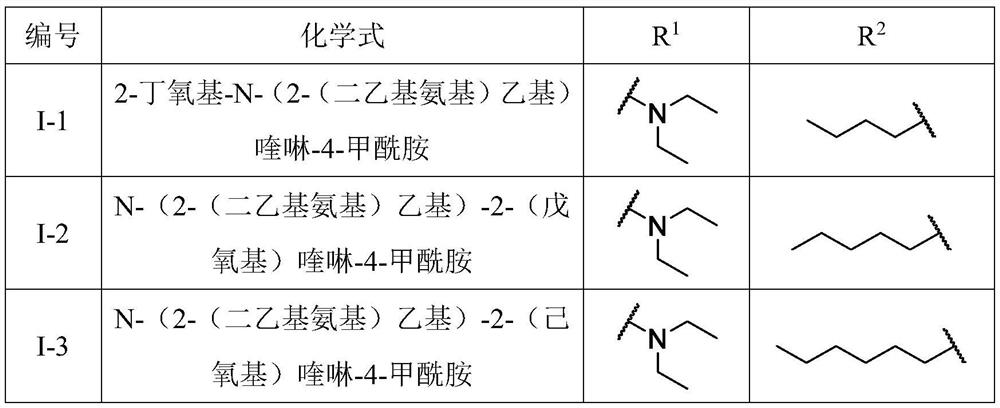
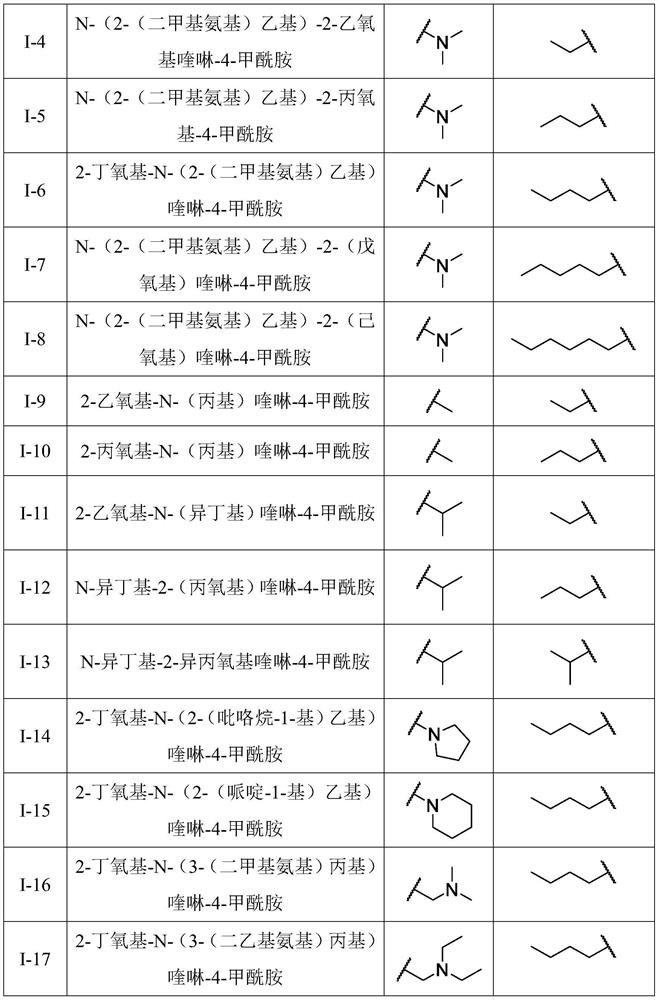
A kind of quinoline carboxamide compound and its preparation method and anti-enterovirus 71 application
A technology of quinoline carboxamide and enterovirus, which is applied in the field of medicine, can solve the problems of stoppage, impossibility of real application, and limited antiviral efficacy, and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] [embodiment 1] the preparation of 2-chloroquinoline-4-formyl chloride II-2
[0028] 2-Chloroquinoline-4-formyl chloride is synthesized by the reaction shown in the following formula i.
[0029]
[0030] 2-Chloroquinoline-4-carboxylic acid (100 mg, 0.482 mmol) was added into a 25 mL round bottom flask, and 5 mL of DCM solution and 2 drops of DMF solution were added while stirring. After fully stirring, 0.088 mL of thionyl chloride solution was added dropwise. After the addition was complete, it was heated to 55° C. and refluxed for 4 hours until the reaction solution became clear. Two drops of the reaction solution were placed in an EP tube, and 0.5 mL of methanol solution was added to quench the reaction, and the progress of the reaction was monitored by TLC. After the reaction was completed, spin dry directly for the next step of reaction.
Embodiment 2
[0031] [Example 2] Preparation of 2-chloroquinoline-4-formamide derivatives II-4a-h
[0032] The 2-chloroquinoline-4-carboxamide derivatives II-4a-h are synthesized by the reaction shown in the following formula ii.
[0033]
[0034] Take the preparation of 2-chloro-N-(2-(diethylamino)ethyl)quinoline-4-carboxamide II-4a as an example: N,N-diethylethylenediamine (61.6mg, 0.530 Add mmol) II-3a into a 25 mL round bottom flask, add 5 mL of DCM and triethylamine solution (0.1 mL, 0.646 mmol) under ice-bath conditions, and stir in ice-bath for 15 minutes. Dissolve the spin-dried 2-chloroquinoline-4-formyl chloride (143.8mg, 0.636mmol) in 2mL of DCM in Example 1, add it dropwise to the original reaction solution under ice bath, remove the ice bath after the dropwise addition, and react at room temperature overnight. After the reaction was completed, the reaction solution was spin-dried and separated by column chromatography to obtain 2-chloro-N-(2-(diethylamino)ethyl)quinoline-4...
Embodiment 3
[0036] [Example 3] Preparation of quinoline carboxamide compound II-6-a-q of the present invention
[0037] The quinoline carboxamide compound is synthesized by the reaction shown in the following formula iii.
[0038]
[0039]
[0040] Sodium hydride (21.2mg, 0.883mmol) was added into a 25mL round bottom flask, argon was introduced into the flask under vacuum, and 3mL DMF was added under ice-bath conditions. Dissolve n-butanol (60.6 mg, 0.818 mmol) in 1 mL of DMF and add it to the original reaction solution. After the addition is complete, remove the ice bath and stir at room temperature for 30 minutes. II-4a (100mg, 0.327mmol) and potassium iodide (54.28mg, 0.327mmol) were dissolved in 1mL DMF respectively, and added to the original reaction solution in sequence. After the addition, react at 80°C for 72 hours. The reaction was monitored by TLC. After the reaction was completed, it was quenched with water, extracted with 3×30 mL of ethyl acetate, washed with 10 mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com