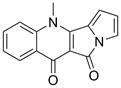
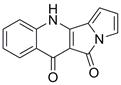
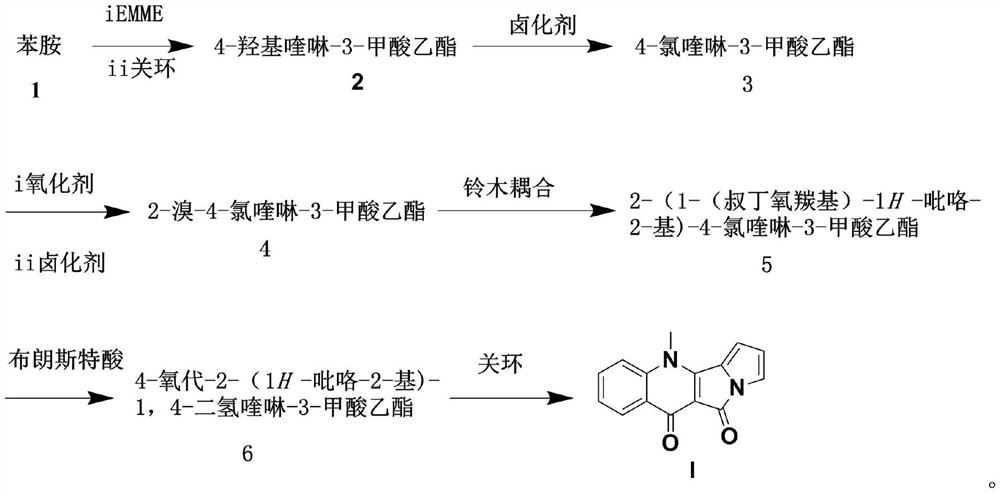
A kind of method for preparing peninoan compound
The technology of a compound and a halogenating agent is applied in the field of synthesis of peninolide compounds, and can solve the problems of high cost, complicated process, cumbersome method steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0031] 4-Hydroxyquinoline-3-carboxylic acid ethyl ester
[0032] Weigh aniline (1.02 mL, 0.011 mol) and diethyl ethoxymethylenemalonate (0.5-2 eq) into ethanol, heat at 100-200°C for three hours, cool to room temperature, add phenetole , heated at 100-200°C for 30min, cooled to room temperature, after the reaction was completed, added ice water, extracted with ethyl acetate (100mL×2), combined the organic phases, washed the organic phases with saturated brine, dried over anhydrous sodium sulfate, and filtered , the organic phase was concentrated, and the residue was subjected to silica gel column chromatography to obtain the product as a white solid 1.6g (76% yield);
[0033] ESI-MS m / z 218.32 [M+H] +
[0034] 1 H NMR (500 MHz, DMSO-d 6 ): 10.62 (brs, 1H), 8.80 (s, 1H), 8.46-7.78(m, 4H), 4.10 (q, J =7.1 Hz, 2H), 1.30 (t, J =7.1 Hz, 3H).
[0035] 4-Chloroquinoline-3-carboxylic acid ethyl ester
[0036] Weigh 4-hydroxyquinoline-3-carboxylic acid ethyl ester (1.6g, 7.3mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com