Chloroquine photoaffinity molecular probe and preparation method and application thereof
A molecular probe, chloroquine light technology, applied in the field of chloroquine photoaffinity molecular probe and its preparation, can solve the problems of poor drug binding ability and inability to visualize enrichment and identification of target proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
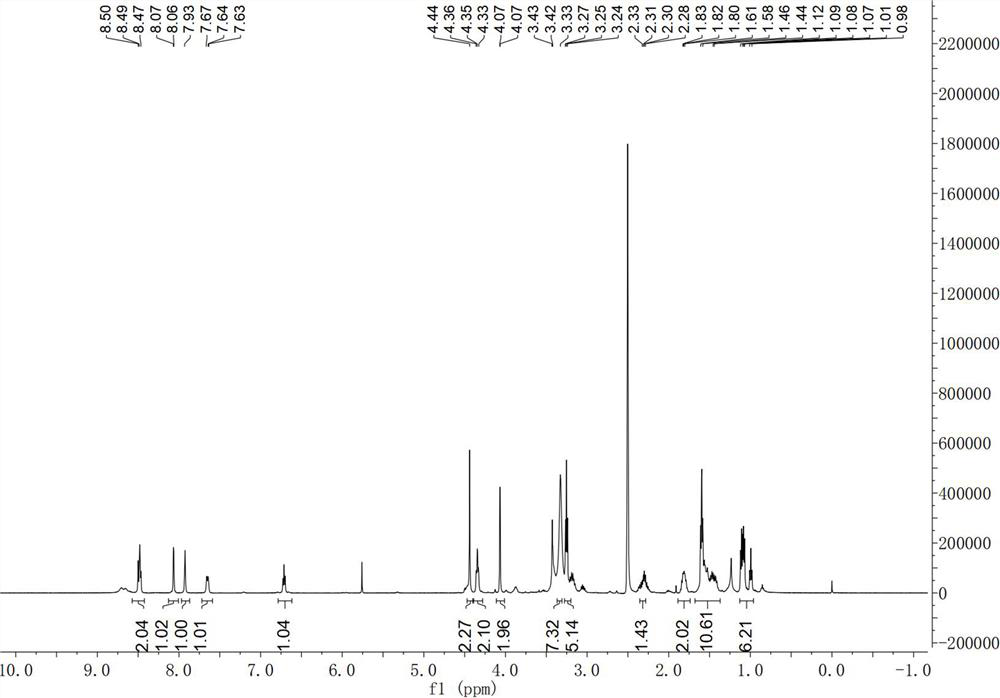
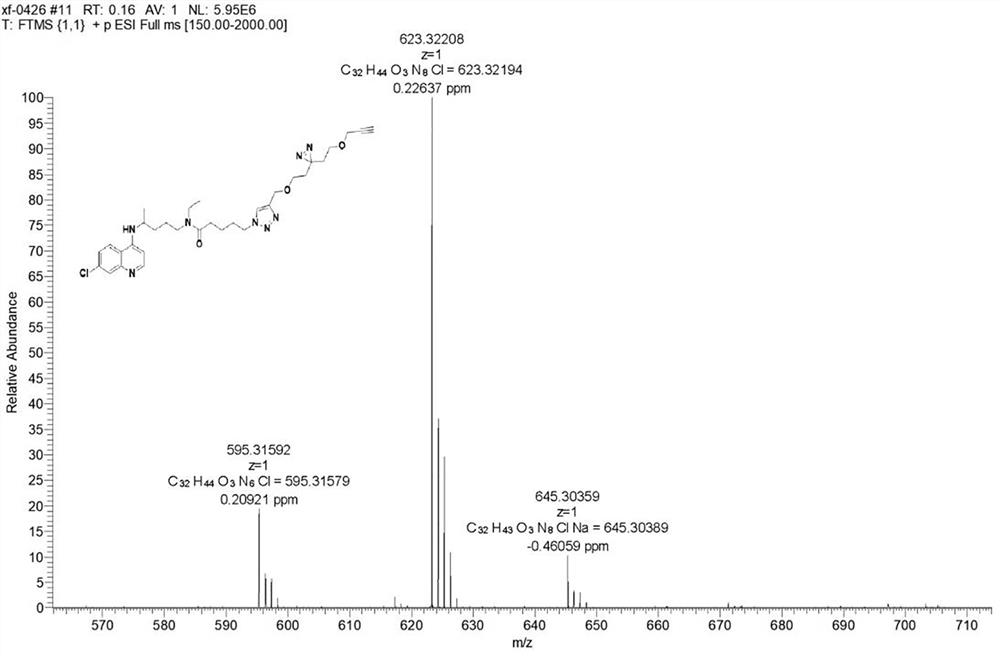
[0025] The first embodiment of the present application discloses a method for preparing a chloroquine photoaffinity molecular probe, comprising the following steps:
[0026] S01. carry out the first nucleophilic substitution reaction with 1,4-dibromopentane and sodium azide, and then carry out hydrogenation reaction to obtain 1,4-diaminopentane;
[0027] S02. The 1,4-diaminopentane and di-tert-butyl dicarbonate are heated and refluxed, and then post-processing is performed to obtain tert-butyl (4-aminopentyl) carbamate;
[0028] S03. The tert-butyl (4-aminopentyl) carbamate, 4,7-dichloroquinoline and dimethyl sulfoxide are subjected to heating reaction, and then deprotection treatment is carried out to obtain N 4 -(7-Chloroquinolin-4-yl)pentane-1,4-diamine;
[0029] S04. Put the N 4 -(7-Chloroquinolin-4-yl)pentane-1,4-diamine reacted with acetaldehyde and then reduced to give N 4 -(7-Chloroquinolin-4-yl)-N 1 -Ethylpentane-1,4-diamine;
[0030] S05. Put the N 4 -(7-Chloro...
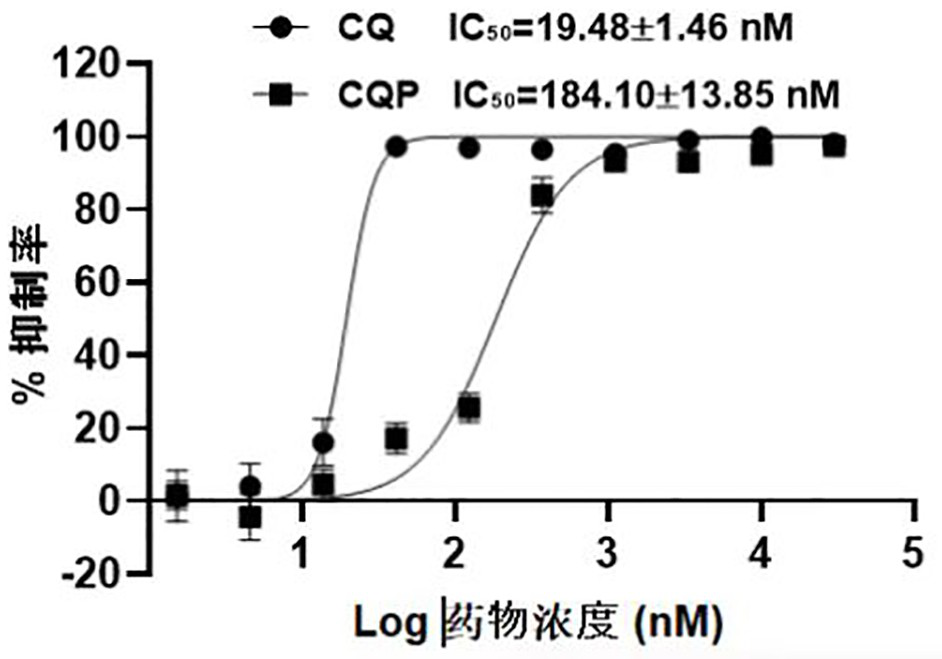
Embodiment 1
[0063] Chloroquine photoaffinity molecular probe and preparation method thereof
[0064] (1) Preparation of 1,4-diazidepentane 2
[0065] ;
[0066] In a 100 mL reaction flask, add sodium azide (4.3 g, 66 mmol), 1,4-dibromopentane (5.1 g, 22 mmol) and 50 mL DMF, and react overnight at 65 °C. After the reaction, 100 mL of water was added to quench, extracted with ethyl acetate (3×100 mL), the organic phase was collected, dried over anhydrous sodium sulfate, filtered, and the organic phase was concentrated in vacuo to obtain a colorless oily 1,4- Pentane diazide in 95% yield.
[0067] (2) Preparation of 1,4-pentanediamine 3
[0068] ;
[0069] In a 250mL reaction flask, add 1,4-diazidepentane (3.2 g, 21 mmol), Pd / C (400 mg) and 150 mL methanol, under H 2 Ambient, overnight reaction. After the reaction was completed, the organic phase was collected by filtration, and concentrated in vacuo to obtain 1,4-diaminopentane with a yield of 92%.
[0070] (3) Preparation of ter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com