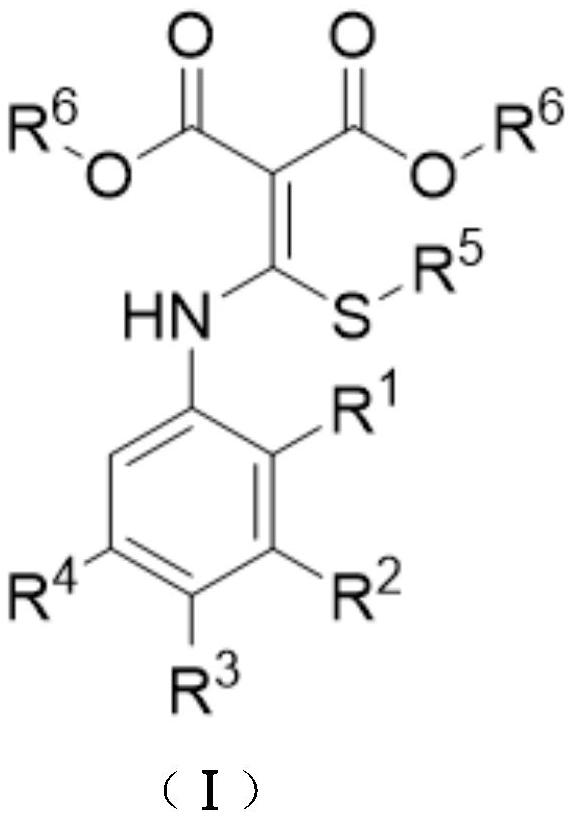
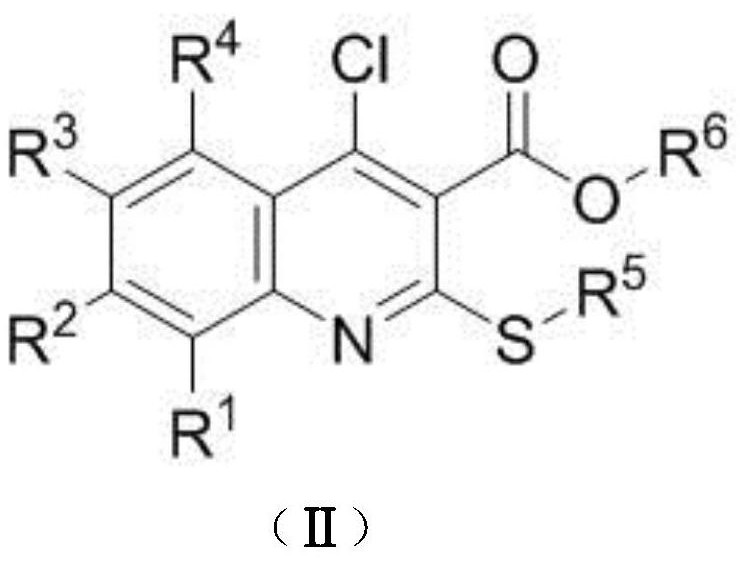
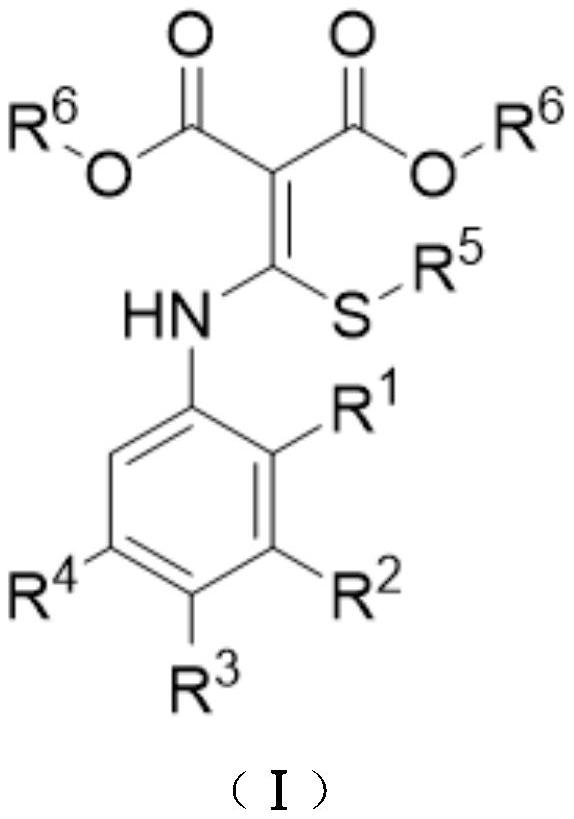
Synthesis method of 4-chloroquinoline compound
A synthesis method and compound technology, which are applied in the field of 4-chloroquinoline compounds and synthesis, can solve the problems of unobtainable raw materials, long reaction time and steps, low total yield and the like, and achieve the effects of low price and shortening synthesis reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Synthesis of 2-ethylthio-4-chloroquinoline-3-methyl formate
[0024] At room temperature, add 13mL chlorobenzene, 0.594g BTC (2.0mmol) to the 15mL thick-walled pressure bottle equipped with a magnetic stirrer, dissolve it at room temperature, add 0.590g substrate 1a (2.0mmol) therein, Seal the reaction system and heat it to 120°C to continue the magnetic stirring reaction for 2.5 hours. After the reaction, the reaction solution is concentrated under reduced pressure, and the obtained concentrate is separated by silica gel column chromatography. First, the volume ratio of petroleum ether and ethyl acetate is 50: The mixed solution of 1 is the eluent, collect the eluent containing the target product, evaporate the solvent and dry to obtain 0.506g light yellow solid product 2-ethylthio-4-chloroquinoline-3-methyl carboxylate 2a, producing The rate is 90%.
[0025] The above reaction formula is as follows:
[0026]
[0027] Product 2-Ethylthio-4-chloroquinolin...
Embodiment 2
[0029] Example 2 Synthesis of 6-methyl-2-ethylthio-4-chloroquinoline-3-formic acid methyl ester
[0030] At room temperature, add 14mL of toluene and 0.594g of BTC (2.0mmol) to a 38mL thick-walled pressure-resistant bottle equipped with a magnetic stirrer, dissolve them at room temperature, and add 0.618g of N,S-ketal ketal 1b (2.0mmol), the reaction system was sealed and heated to 120°C to continue the magnetic stirring reaction for 3h. After the reaction was over, the reaction solution was concentrated under reduced pressure, and the resulting concentrate was separated by silica gel column chromatography. A 50:1 mixture was used as the eluent, the eluate containing the target product was collected, the solvent was evaporated and dried to obtain 0.473g of a white solid product 6-methyl-2-ethylthio-4-chloroquinoline-3 - Methyl formate 2b in 80% yield.
[0031] The above reaction formula is as follows:
[0032]
[0033] Product 6-Methyl-2-ethylthio-4-chloroquinoline-3-carb...
Embodiment 3
[0035] Example 3 Synthesis of 6-methylthio-2-ethylthio-4-chloroquinoline-3-formic acid methyl ester
[0036] At room temperature, add 10 mL of xylene and 0.712 g of BTC (2.4 mmol) to a 15 mL thick-walled pressure-resistant bottle equipped with a magnetic stirrer, dissolve them at room temperature, and add 0.682 g of N,S-ketal 1c (2.0mmol), the reaction system was sealed and heated to 120°C to continue the magnetic stirring reaction for 3h. After the reaction was over, the reaction solution was concentrated under reduced pressure, and the resulting concentrate was separated by silica gel column chromatography. The mixture of 70:1 is the eluent, the eluate containing the target product is collected, the solvent is evaporated and dried to obtain 0.577g yellow solid product 6-methylthio-2-ethylthio-4-chloroquinoline- Methyl 3-carboxylate 2c, 88% yield.
[0037] The above reaction formula is as follows:
[0038]
[0039] Product 6-Methylthio-2-ethylthio-4-chloroquinoline-3-carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com