Triazolotetrazine compound containing morpholine and quinoline rings as well as preparation method and application thereof
A compound and quinoline ring technology, applied in the field of drug synthesis, can solve the problems of less anti-tumor activity and unpredictable drug activity of unknown compounds, and achieve the effects of easy availability of reaction reagents, excellent biological activity, and fewer by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
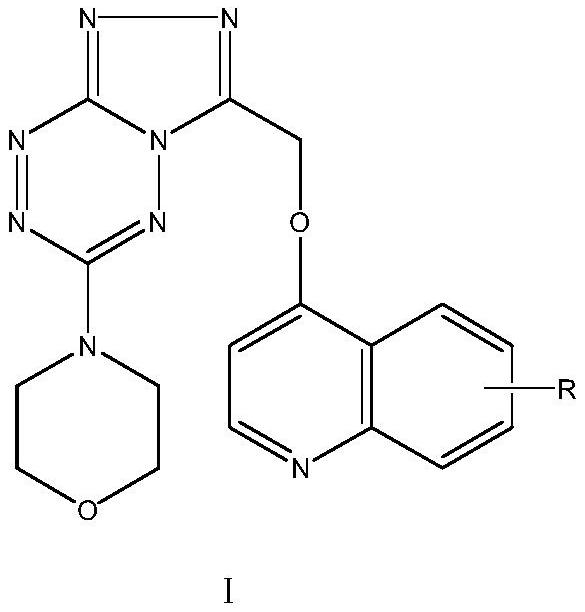
[0033] The triazolotetrazine compound containing morpholine and quinoline rings of the present invention is characterized in that the structural formula of the compound is as shown in formula I below:
[0034]
[0035] In the above formula I, the substituent R is selected from hydrogen, halogen, alkoxy, benzyloxy or trihaloalkyl, where the halogen can be chlorine, bromine or fluorine, preferably substituted by fluorine, alkoxy long-chain alkoxy A group such as an alkoxy group with more than 5 C atoms or a short-chain alkoxy group is good C 1 -C 4 Alkoxy groups, such as methoxy, ethoxy or propoxy groups; trihaloalkyl groups are preferably trifluoromethyl, trifluoroethyl and other groups. The compounds screened above all have good anti-tumor activity and inhibit the activity of tumor cells, so as to achieve the expected goal of prevention or treatment. Compared with the antitumor activity of cisplatin, it can also achieve comparable or better activity performance. For abov...
Embodiment 2
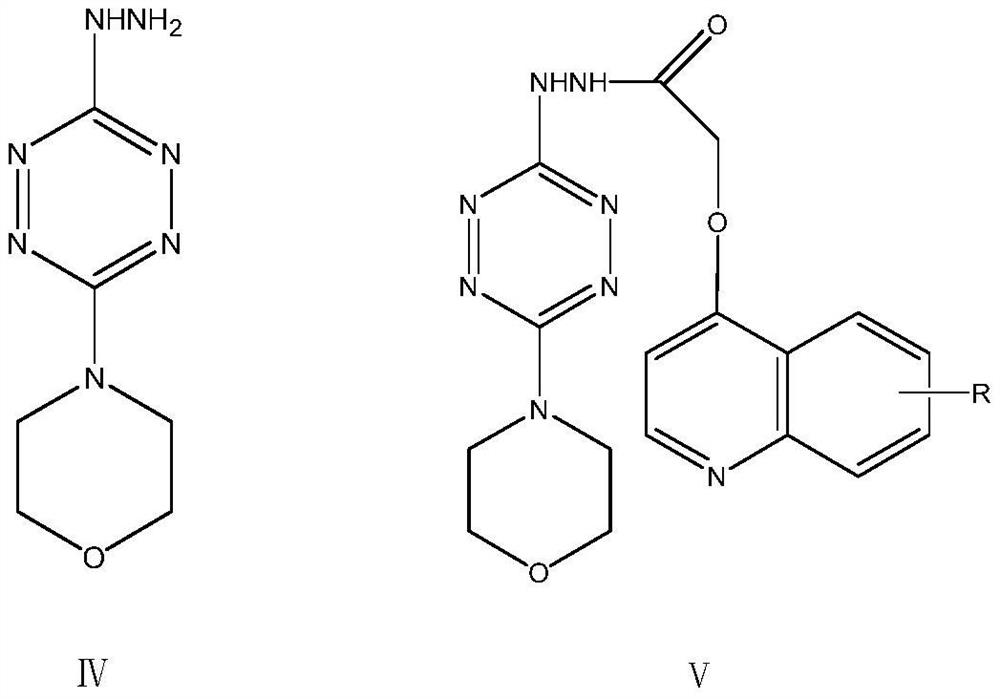
[0045] The compound of this example is 4-(3-((quinoline-4-oxyl)methyl)-1,2,4]triazol[4,3-b][1,2,4,5]tetra Oxyzin-6-yl) morpholine, its structural formula is shown in following formula I-1:
[0046]
[0047] The synthesis of the above compounds is shown below:
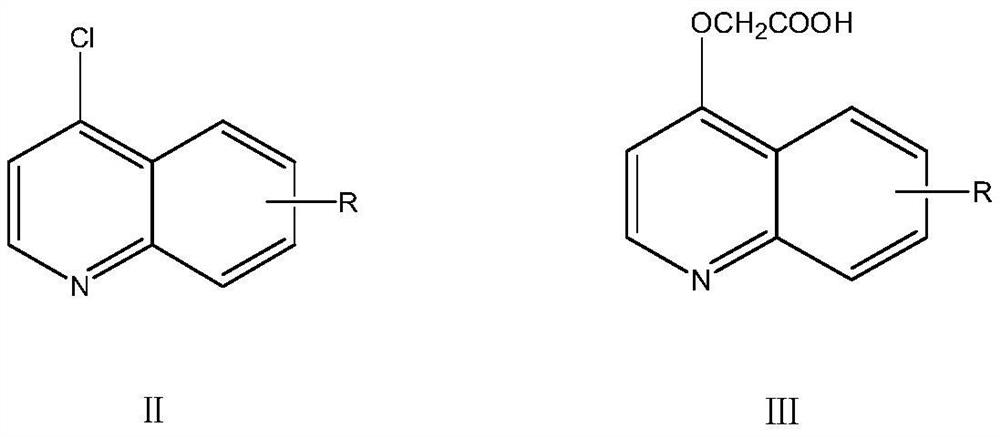
[0048] Synthesis of intermediate 2-(quinoline-4-propoxy)acetic acid:
[0049] Add 2.47g (32.5mmol) of glycolic acid and 2.52g (45mmol) of potassium hydroxide into a 100mL three-necked flask, heat up to 170°C for sufficient reflux reaction, and then pre-dissolve 2.13g (13mmol) of 4-chloroquinoline in anhydrous After DMSO, the corresponding DMSO solution containing 4-chloroquinoline was formulated, and the DMSO solution containing 4-chloroquinoline was slowly added dropwise to the reaction solution. During the dropping process, the temperature of the reaction system was controlled at 165°C to 170°C. Finally, keep the temperature for heat preservation reaction for 2.5 hours. After the reaction, remove the reaction sol...
Embodiment 3
[0067] The compound of this example is 4-(3-((6-methoxyquinolin-4-yl)oxy)methyl)-[1,2,4]triazol[4,3-b][1 , 2,4,5] tetrazin-6-yl) morpholine, its structural formula is as shown in the following formula I-2:
[0068]
[0069] The synthesis of above-mentioned corresponding compound is as follows:
[0070] Synthesis of intermediate product 2-((6-methoxyquinolin-4-yl)oxy)acetic acid:
[0071] Add 24.7g (325mmol) of glycolic acid and 25.2g (450mmol) of potassium hydroxide into a 1000mL three-necked flask, heat up to about 170°C for sufficient reflux reaction, and then add 25.2g of 4-chloro-6-methoxyquinoline (130mmol) was dissolved in anhydrous DMSO in advance to form a DMSO solution containing 4-chloro-6-methoxyquinoline, and the solution was slowly added dropwise to the reaction solution, and the dropwise process controlled the temperature of the reaction system at 165° C. 170°C. After the dropwise addition, keep the temperature for heat preservation reaction for 2.5 hours. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com