Ursdic acid isatin amide derivatives as well as preparation method and application thereof
A technology of indole quinone amide and ursolic acid, which is applied in the direction of drug combination, steroids, anti-tumor drugs, etc., can solve the problem of lack of anti-tumor activity and achieve the effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
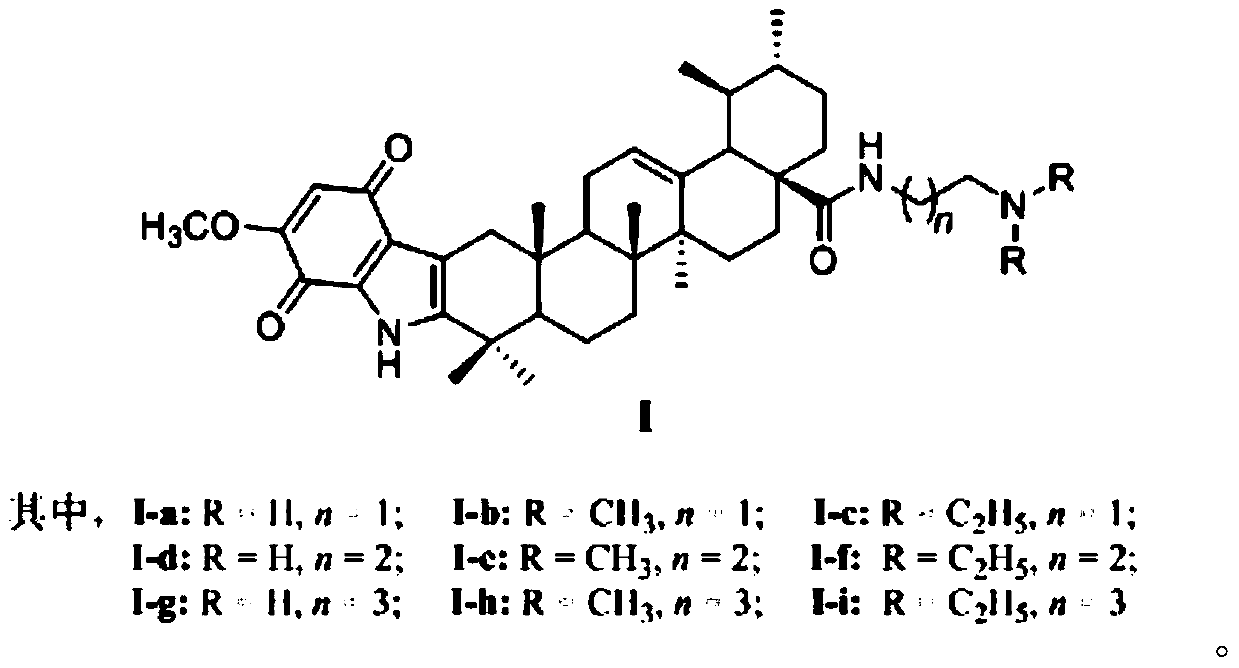
[0043] The preparation method of the ursolic acid indole quinone amide derivative I-a to I-i of structure shown in general formula (I) of the present invention comprises the steps:
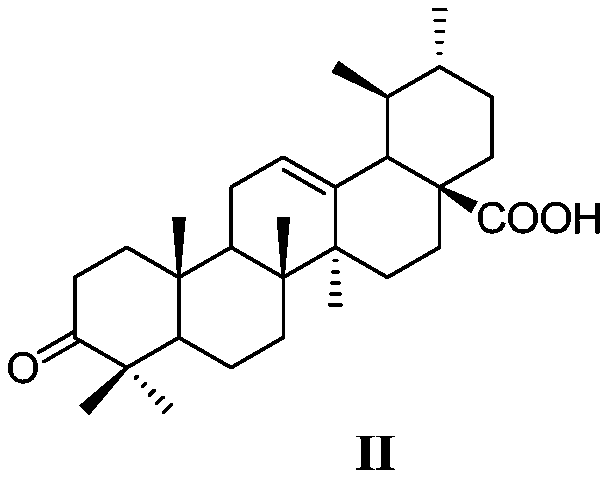
[0044] (1) ursolic acid obtains 3-oxidized ursolic acid through Jones reagent oxidation reaction, has the structure shown in general formula II:
[0045]
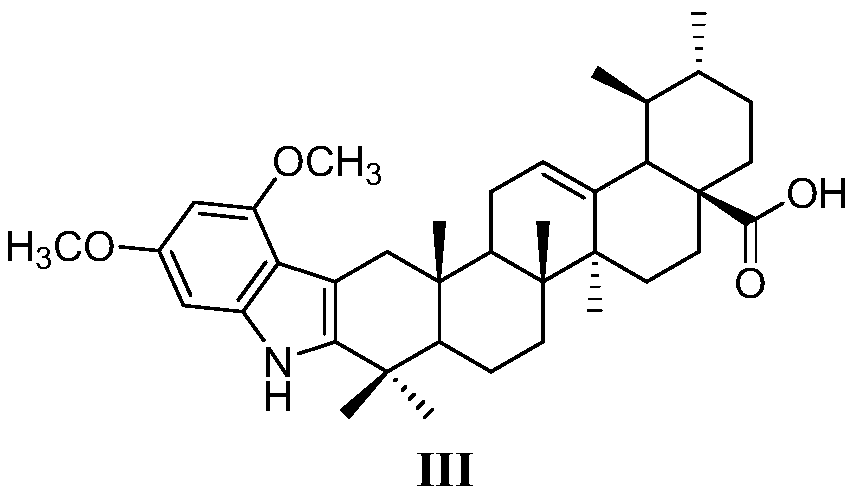
[0046] (2) 3-oxidized ursolic acid and 3,5-dimethoxyphenylhydrazine are synthesized by Fishier indole to obtain 3,5-dimethoxyindole derivatives, which have the structure shown in the general formula III:
[0047]
[0048] (3) Vilsmeier-Haack formylation of indole derivatives with phosphorus oxychloride and N,N-dimethylformamide to obtain 3,5-dimethoxy-2-formyl indole derivatives, Has the structure shown in general formula IV:
[0049]
[0050] (4) compound IV is carried out oxidation reaction with hydrogen peroxide, obtains ursolic acid indole quinone derivative, has the structure shown in general formula V:
[0051] (5) Compound V...
Embodiment 13
[0067] The synthesis of embodiment 13-oxidized ursolic acid (II) of the present invention
[0068]Add 2g (4.6mmol) of ursolic acid and 250mL of acetone into a 500mL round bottom flask, stir to dissolve, stir in ice water for 15min, slowly add 1.87mL of Jones reagent dropwise and rise to room temperature, stir for 5h, then add 90mL Virahol stirring reaction 30min, after reaction finishes, filter and precipitate and collect filtrate, the light yellow-green viscous solid that filtrate concentrating under reduced pressure obtains is 3-oxidized ursolic acid (II) ( 1.2 g, 65.6%).
Embodiment 2
[0070] Synthesis of 3,5-Dimethoxindole Derivative of Ursolic Acid (III)
[0071] Weigh 0.33 g (3 mmol) of 3,5-dimethoxyaniline and dissolve it in 3 mL of 20% hydrochloric acid in a single-necked round bottom flask. Then weigh 0.28 g (4 mmol) of sodium nitrite and dissolve it in 0.7 mL of water, slowly drop it into the reaction flask under ice-bath conditions, and stir and react under ice-bath conditions for 1 h. Weigh 1.35g (6mmol) of tin protochloride and dissolve it in 1.8mL of concentrated hydrochloric acid, slowly drop it into the flask where the previous step reaction under ice-bath conditions is completed, stir at room temperature for 2 hours, carry out suction filtration after the reaction is completed, and the solid is 3,5-Dimethoxyphenylhydrazine.
[0072] Weigh 0.441g (1mmol) of 3-oxidized ursolic acid and dissolve it in 10mL of absolute ethanol, add 3,5-dimethoxyphenylhydrazine (3mmol) from the previous step into the system, and then add 0.5mL of concentrated hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com