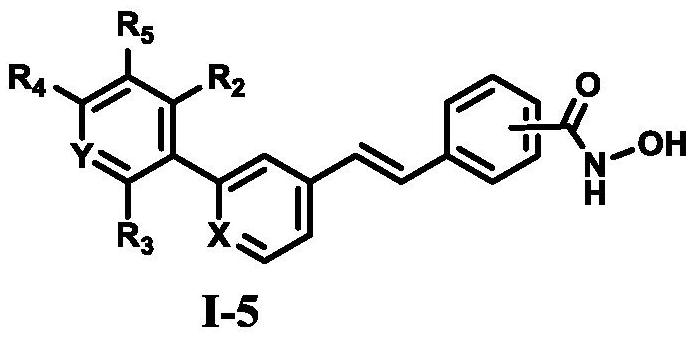
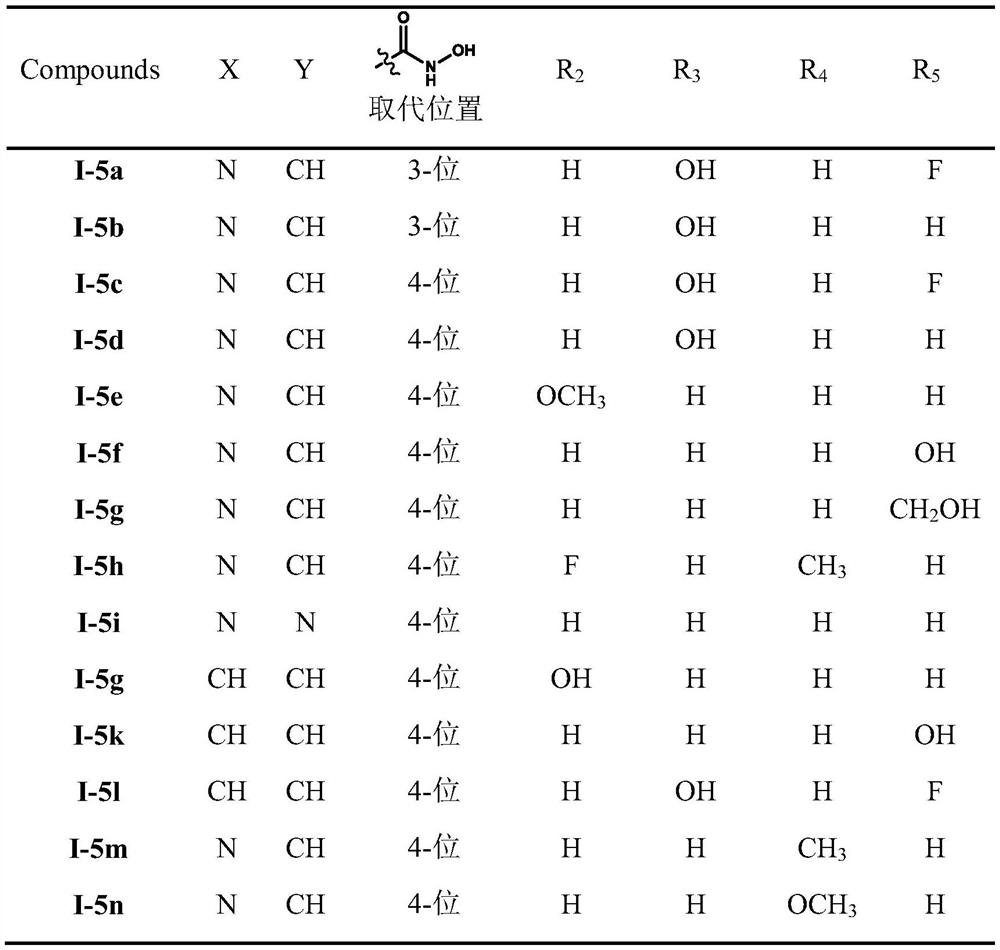
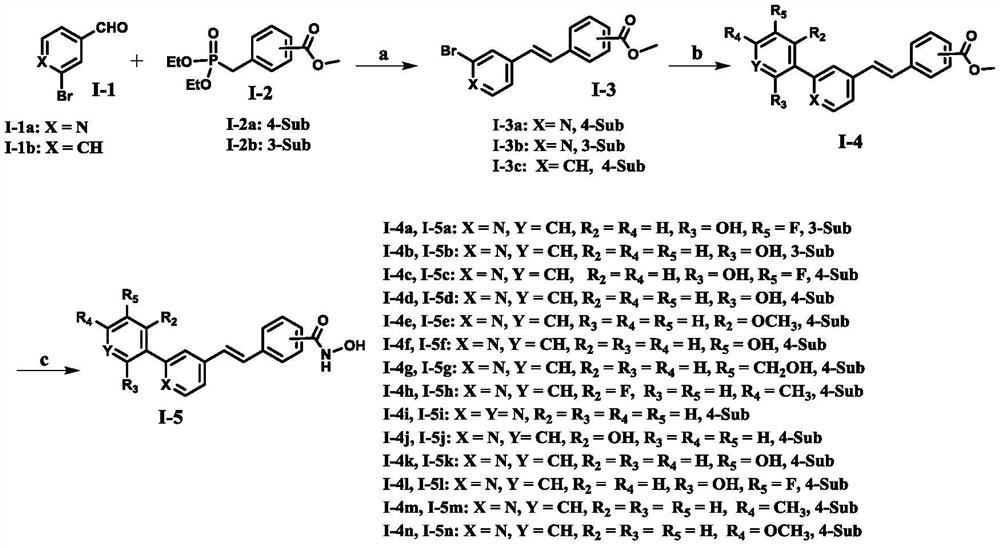
A class of diarylethene lsd1/hdacs dual-target inhibitors containing hydroxamic acid group, preparation method and application thereof
A technology of diarylethene and hydroxamic acid, which is applied in the field of application of diarylethene LSD1/HDACs dual-target inhibitors in the preparation of anti-tumor drugs, to achieve good in vitro anti-tumor activity, good selectivity, and effective The effect of promoting the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 (E)-Synthesis of methyl 4-(2-(2-bromo-pyridin-4-yl)alkenyl)benzoate (I-3a)
[0023]
[0024] Compound I-1a (376 mg, 2.0 mmol) and I-2a (601.1 mg, 2.1 mmol) were added to a 50-mL two-necked flask, dissolved in 10 mL of anhydrous DMF, and t-BuOK (673.3 mg, 6.0 mmol) was added under ice bath. After the addition was completed, the reaction was stirred at room temperature for 0.5 hours, and then the reaction system was slowly added to ice water (50 mL), and a solid was precipitated. The solid was collected by suction filtration, separated and purified by silica gel column chromatography (petroleum ether:ethyl acetate=5:1) to obtain 396.4 mg of compound I-3a, white solid, yield: 62.3%, Mp: 106-107°C. 1 H NMR (400 MHz, CDCl 3 )δ8.36(d,1H,J=5.2Hz),8.08(d,2H,J=8.4Hz),7.60-7.57(m,3H),7.34-7.29(m,2H),7.04(d,1H) , J=16.4Hz), 3.95(s, 3H). 13 CNMR (101MHz, CDCl 3 )δ166.57,150.44,146.96,143.04,139.95,133.46,130.39,130.20,127.05, 126.87,125.16,120.09,52.27.HRMS(ESI)cal...
Embodiment 2
[0025] Example 2 (E)-Synthesis of methyl 4-(2-(2-bromo-pyridin-4-yl)alkenyl)benzoate (I-3b)
[0026]
[0027] According to the method of Example 1, compound I-2b (601.1 mg, 2.1 mmol) was used to replace I-2a to obtain the target compound I-3b 288.3 mg, white solid, yield: 45.4%, Mp: 73-74°C. 1 H NMR (400MHz, CDCl 3 )δ8.33(d,1H,J=5.2Hz),8.21(t,1H,J=2.0Hz),8.00(dt,1H,J 1 =1.2Hz, J2 =8.0Hz),7.70(dt,1H,J 1 =1.6Hz,J 2 =8.0Hz), 7.57(s, 1H), 7.48(t, 1H, J=8.0 Hz), 7.35-7.30(m, 2H), 7.03(d, 1H, J=16.4Hz), 3.96(s, 3H ).HRMS(ESI)calcd for C 15 H 12 BrNNaO 2 [M+Na] + :339.9944,Found:339.9943.
Embodiment 3
[0028] Example 3 (E)-Synthesis of methyl 3-(3-bromostyryl) formate (I-3c)
[0029]
[0030] According to the method of Example 1, compound I-1b (370 mg, 2.0 mmol) was used to replace I-1a to obtain the target compound I-3c 480 mg, yield: 75.6%, Mp: 177-178 ° C. 1 H NMR (400MHz, DMSO-d 6 ) δ7.97(d,2H,J=8.4Hz),7.89(t,1H,J=2.0Hz),7.74(d,2H,J=8.4Hz),7.64(dt,1H,J 1 =1.2Hz,J 2 =8.0Hz), 7.51-7.48(m, 1H), 7.43(d, 2H, J=4.0Hz), 7.37(t, 1H, J=8.0Hz), 3.86(s, 3H). 13 C NMR (101MHz, DMSO-d 6 )δ166.41,141.90,139.66, 131.31,131.20,130.09,129.65,129.38,129.04,127.28,126.38,122.76,52.57. HRMS(ESI)calcd for C 16 H 13 BrNaO 2 [M+Na] + :338.9991,Found:338.9996.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com