Oxonantenine rare-earth complex as well as synthesis method and application thereof
A technology of rare earth complexes and nandinaphylline, which is applied in the field of oxidized nandinaphylline rare earth complexes and its synthesis, can solve blank problems and achieve good in vitro antitumor activity and superior in vitro antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Synthesize ONT-Nd with normal pressure solution method Ⅲ Complex
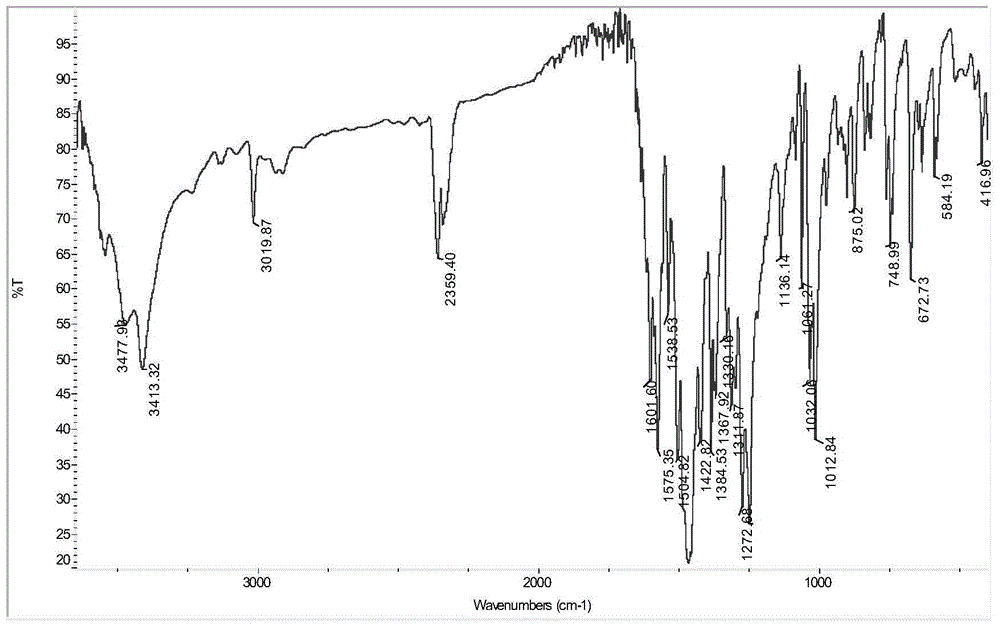
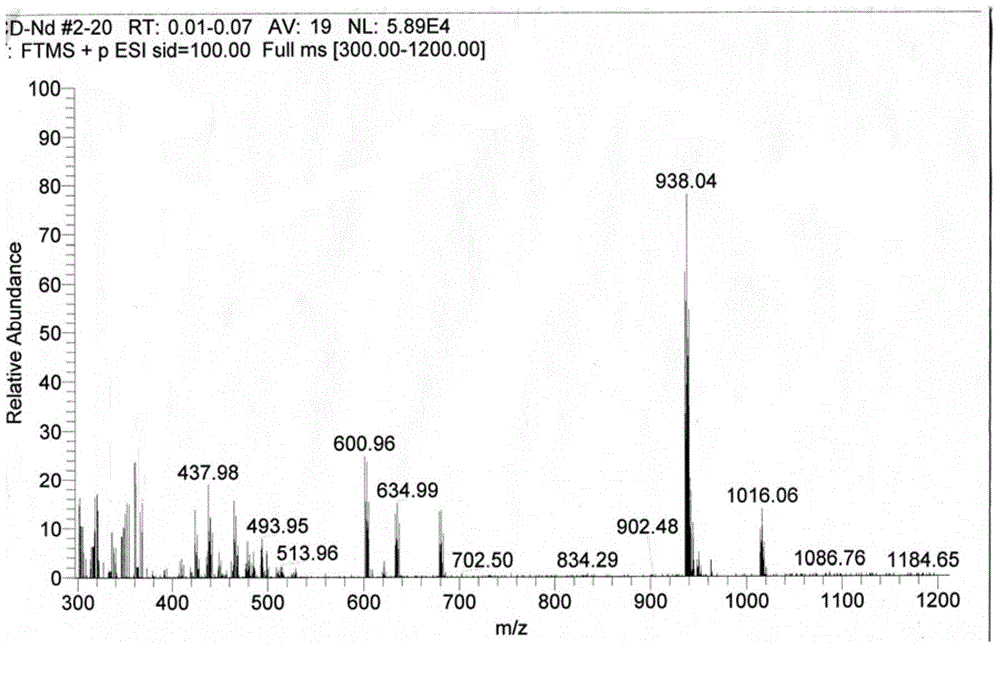
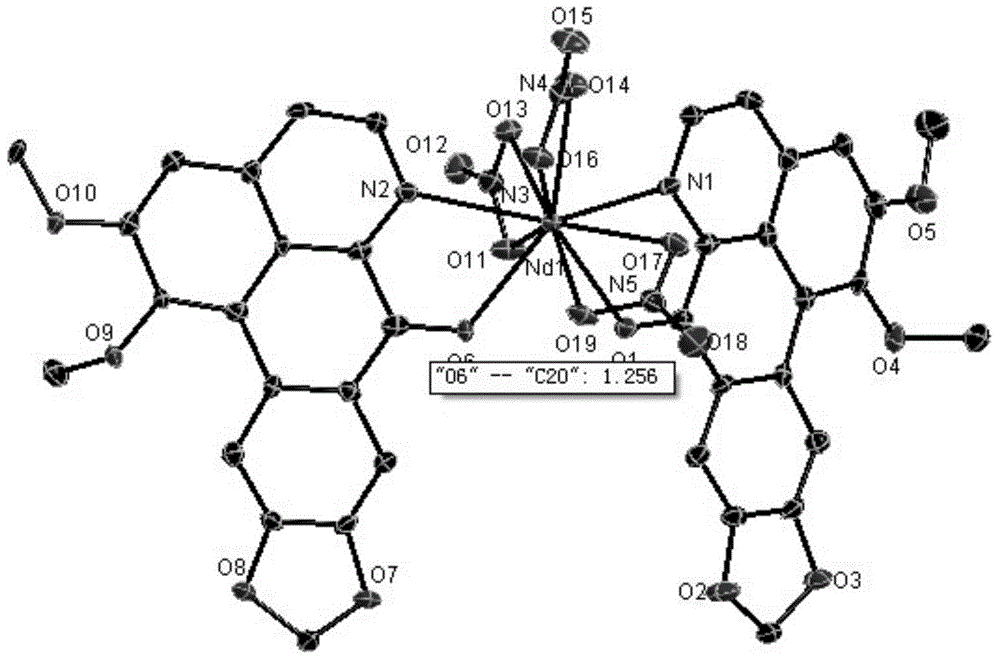
[0040] Take 1mmol Nd(NO 3 ) 3 .6H 2 O was dissolved in 5mL of methanol; 2mmol of ONT was heated and dissolved in 15mL of methanol. The two solutions were mixed and reacted at 70°C (reflux) for 1 hour. After the reaction, a red suspension was obtained with a large number of complexes. A solid was formed; the solution was filtered, and the filtered solid was washed with water, methanol, and ether in turn, and dried to obtain a dark red solid product. The crimson product that this embodiment gained is through infrared spectrum (collection as shown in FIG. figure 1 shown), high-resolution mass spectrometry (spectrum as shown in figure 2 shown) and X single crystal diffraction (pattern as image 3 Shown) analysis, confirm that the dark red solid product obtained is the target complex [Nd(ONT) 2 (NO 3 ) 3 ].
Embodiment 2
[0041] Embodiment 2: Synthesize ONT-Gd with normal pressure solution method Ⅲ Complex
[0042] 1mmol Gd(NO 3 ) 3 .6H 2 O was dissolved in 10mL of water; 2mmol of ONT was dissolved in 50mL of ethanol. The two solutions were mixed and reacted at 60°C (reflux) for 10 hours. After the reaction, a red suspension was obtained, and a large amount of complex solids were formed. ; The solution was filtered, and the filtered solid was washed with water, ethanol and ether in turn, and dried to obtain a red solid product. The crimson product that this embodiment gained is through infrared spectrum (collection as shown in FIG. Figure 4 shown), electrospray mass spectrometry (spectrum as shown in Figure 5 shown) and X single crystal diffraction (pattern as Figure 6 Shown) analysis, it is determined that the obtained deep red solid product is the target complex [Gd(ONT) 2 (NO 3 ) 3 ].
Embodiment 3
[0043] Embodiment 3: Synthesize ONT-Tb with normal pressure solution method Ⅲ Complex
[0044] 1mmol Tb(NO 3 ) 3 .6H 2 O was dissolved in 50mL of water; 2mmol of ONT was dissolved in 200mL of acetone, and the two solutions were mixed and reacted at 80°C (reflux for condensation) for 20 hours. After the reaction, a red clear solution was obtained; most of them were removed by distillation under reduced pressure Solvent, a large amount of complex solids were precipitated, after cooling, the solution was filtered, and the filtered solids were washed with water, ethanol, and ether in turn, and dried to finally obtain a red solid product. The crimson product that this embodiment gained is through infrared spectrum (collection as shown in FIG. Figure 7 shown), high-resolution mass spectrometry (spectrum as shown in Figure 8 shown) and X single crystal diffraction (pattern as Figure 9 Shown) analysis, it is determined that the dark red solid product obtained is the target co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com