Fluorine-substituted diphenylethane compound and preparation method and application thereof
A technology of diphenylethane and compounds, applied in the field of drug synthesis, can solve the problems of harsh reaction conditions, tumor cell apoptosis, and many by-products, and achieve simple and easy preparation conditions, strong in vitro anti-tumor activity, and few by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
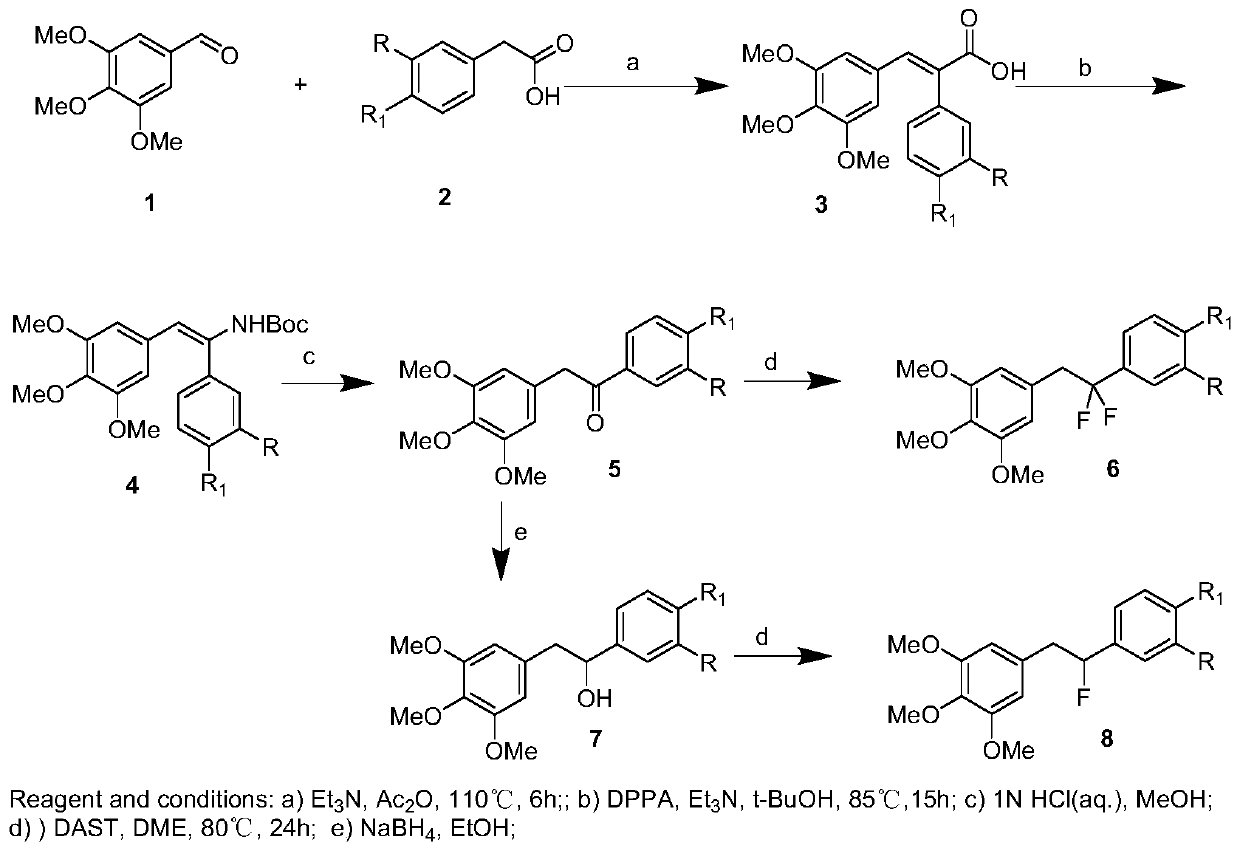
[0042] Synthesis of (E)-2-(4-methoxyphenyl)-3-(3,4,5-trimethoxyphenyl)acrylic acid (3a), see figure 1 :
[0043]Dissolve 3,4,5-trimethoxybenzaldehyde (7.9g, 40mmol) and p-methoxyphenylacetic acid (6.7g, 40mmol) in 150mL of acetic anhydride, then add triethylamine (8.1g, 80mmol) , The mixture was heated at 110° C. for 6 h. After cooling, it was acidified with concentrated hydrochloric acid, poured into ice water, and stirred for 4 hours to obtain a light yellow solid, which was filtered, dissolved in 10% NaOH aqueous solution, washed with ethyl acetate to decolorize, separated the organic layer, and kept the aqueous phase. Hydrochloric acid was added to the aqueous phase until pH = 3-4. The precipitated solid was filtered and recrystallized from ethyl acetate to obtain 3a, 11.0 g, yield 80.2%, white solid, mp: 188.6-189.3°C.
[0044] 1 H NMR (500MHz, CDCl 3 ): δ7.82(s,1H),7.20(d,J=10.0Hz,2H),6.96(d,J=5.0Hz,2H),6.37(s,2H),3.81(s,6H),3.58 (s,6H);
[0045] 13 C NMR (125MHz...
Embodiment 2
[0078] Preparation of 1-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)ethan-1-ol (7a), see figure 1 :
[0079] 5a (1.6 g, 5 mmol) was dissolved in 30 mL of absolute ethanol, and NaBH was added in portions at 0 °C 4 (0.3g, 7.5mmol), stirred for half an hour, returned to room temperature and reacted overnight. Quenched with water, extracted with ethyl acetate, combined organic phases, dried over anhydrous sodium sulfate, filtered, and spin-dried, the residue was purified by silica gel chromatography to obtain 7a (1.0 g, yield 65.0%), which was directly used in the next reaction.
[0080] Preparation of 5-(2-fluoro-2-(4-methoxyphenyl)ethyl)-1,2,3-trimethoxybenzene (8a):
[0081] It is one of the fluorine-substituted diphenylethane compounds in the present invention.
[0082] Dissolve 7a in dichloromethane, add DAST (0.8g, 4.7mmol) dropwise at 0°C and stir for half an hour, return to room temperature, stir for 18h, TLC monitors that the reaction is complete, drop ice water to que...
Embodiment 3
[0084] For the preparation of 1-(3-(benzyloxy)-4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)ethan-1-ol (7b), see figure 1 :
[0085] 5b (1.6g, 5mmol) was dissolved in 30mL of absolute ethanol, NaBH4 (0.3g, 7.5mmol) was added in batches at 0°C, stirred for half an hour, returned to room temperature and reacted overnight. Quenched with water, extracted with ethyl acetate, combined organic phases, dried over anhydrous sodium sulfate, filtered, and spin-dried, the residue was purified by silica gel chromatography to obtain 7b (1.3 g, yield 64.5%), which was directly used in the next reaction.
[0086] Preparation of 5-(1-fluoro-1-(3-(benzyloxy)-4-methoxyphenyl)ethyl)-1,2,3-trimethoxybenzene (8c), which is the subject of the present invention One of the fluorine-substituted diphenylethane compounds. see figure 1 .
[0087] Dissolve 7b in dichloromethane, add DAST (0.8g, 4.7mmol) dropwise at 0°C and stir for half an hour, return to room temperature, stir for 18h, TLC monitors that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com