Tripterine derivate and use thereof
A technology of tripterycin and its derivatives, which is applied in the direction of drug combinations, steroidal compounds, and medical preparations containing active ingredients, etc., and can solve the problem of failure to explain the content of tripteryne and the failure to explain the effectiveness of tripteryne. Composition etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
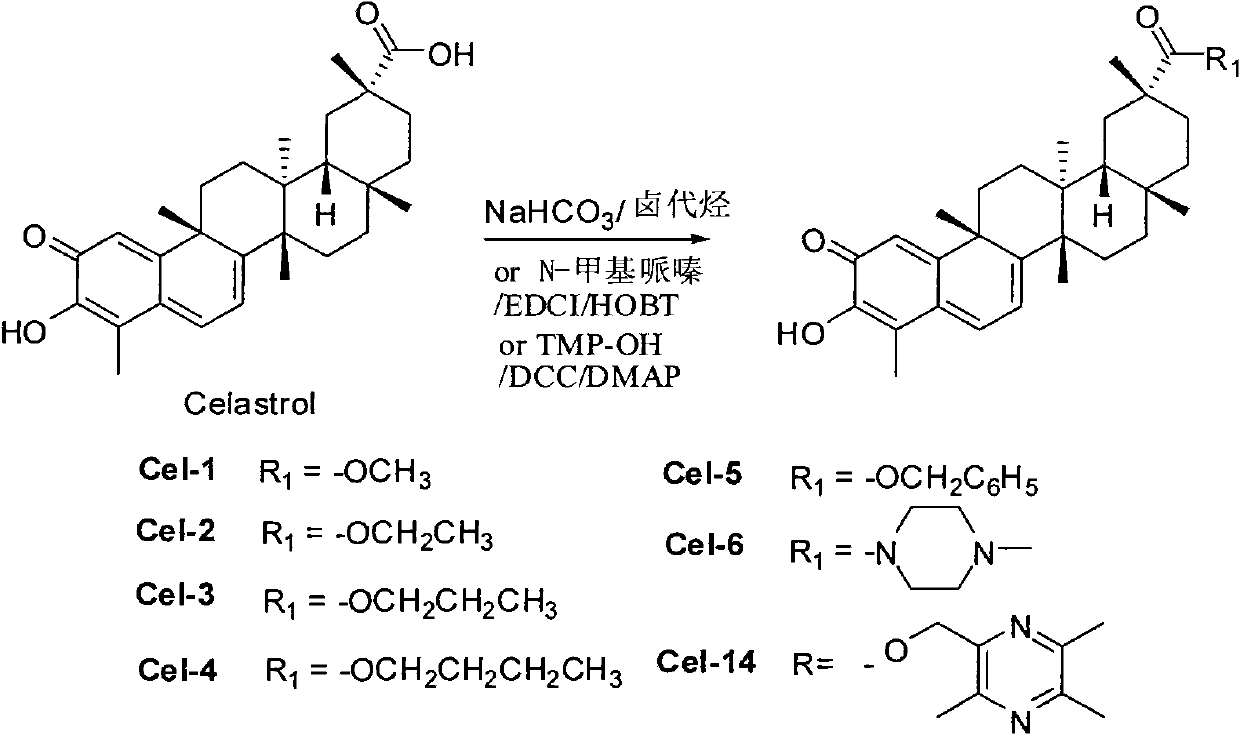
[0040] Embodiment 1 prepares tripterine methyl ester (synthetic route figure such as figure 1 shown)
[0041] Dissolve tripterine (20mg, 0.044mmol) in N,N-dimethylformamide (1ml) and stir to dissolve, add NaHCO 3 (19 mg, 0.22 mmol), followed by the addition of iodomethane (0.22 mmol). Stir the reaction at room temperature for 2 hours, stop the reaction and add deionized water (15ml), extract 3 times with ethyl acetate, combine the ethyl acetate layer, then wash the ethyl acetate layer 3 times with saturated NaCl, and wash with anhydrous NaCl 2 SO 4 Dry and concentrate with a rotary evaporator to obtain a dark red oil. The crude product was separated and purified by flash column chromatography (ethyl acetate: n-hexane), and the product was dried in vacuo to obtain 17 mg of dark red solid Cel-1 with a yield of 83%.
[0042] The mass spectrometric analysis of compound Cel-1 is as follows: MS(EI)[M+H] + m / z 465.6.MS(EI)[M+Na-H] + m / z 487.4. 1 H NMR (CDCl 3 , 400MHz): δ7.0...
Embodiment 2
[0043] Embodiment 2 prepares tripterine ethyl ester (synthetic route figure is as follows figure 1 shown)
[0044] Dissolve tripterine (20mg, 0.044mmol) in N,N-dimethylformamide (1ml) and stir to dissolve, add NaHCO 3 (19 mg, 0.22 mmol), followed by bromoethane (0.22 mmol). Stir the reaction at room temperature for 24 hours, stop the reaction and add deionized water (15ml), extract 3 times with ethyl acetate, combine the ethyl acetate layer, then wash the ethyl acetate layer 3 times with saturated NaCl, anhydrous NaCl 2 SO 4 Dry and concentrate with a rotary evaporator to obtain a dark red oil. The crude product was separated and purified by flash column chromatography (ethyl acetate: n-hexane), and the product was dried in vacuo to obtain 19 mg of dark red solid Cel-2 with a yield of 90%.
[0045] The mass spectrometric analysis of compound Cel-2 is as follows: MS(EI)[M+H] + m / z 479.9. 1 H NMR (CDCl 3 , 400MHz): δ7.03 (1H, dd, J=1.3, 7.1Hz, H-6), 6.56 (1H, d, J=1.4Hz, ...
Embodiment 3
[0046] Embodiment 3 prepares tripterine propyl ester (synthetic route figure is as follows figure 1 shown)
[0047] Dissolve tripterine (20mg, 0.044mmol) in N,N-dimethylformamide (1ml) and stir to dissolve, add NaHCO 3 (19 mg, 0.22 mmol), followed by bromopropane (0.22 mmol). Stir the reaction at room temperature for 24 hours, stop the reaction and add deionized water (15ml), extract 3 times with ethyl acetate, combine the ethyl acetate layer, then wash the ethyl acetate layer 3 times with saturated NaCl, anhydrous NaCl 2 SO 4 Dry and concentrate with a rotary evaporator to obtain a dark red oil. The crude product was separated and purified by flash column chromatography (ethyl acetate:n-hexane), and the product was dried in vacuo to obtain 16 mg of dark red solid Cel-3 with a yield of 74%.
[0048] The mass spectrometric analysis of compound Cel-3 is as follows: MS(EI)[M+H] + m / z493.6. 1 H NMR (CDCl 3 , 400MHz): δ7.04 (1H, dd, J=1.4, 7.1Hz, H-6), 6.60 (1H, d, J=1.4Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com