Quinoline fluorescent compound, preparation method and application thereof
A fluorescent compound and compound technology, applied in the field of fluorescent dyes, can solve the problems of low excitation wavelength, short excitation and emission wavelength, and limit the application of quinoline, and achieve the effects of large displacement value, high quantum yield, and excellent fluorescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of 2-Chloro-7-diethylaminoquinoline-3-carbaldehyde (1)
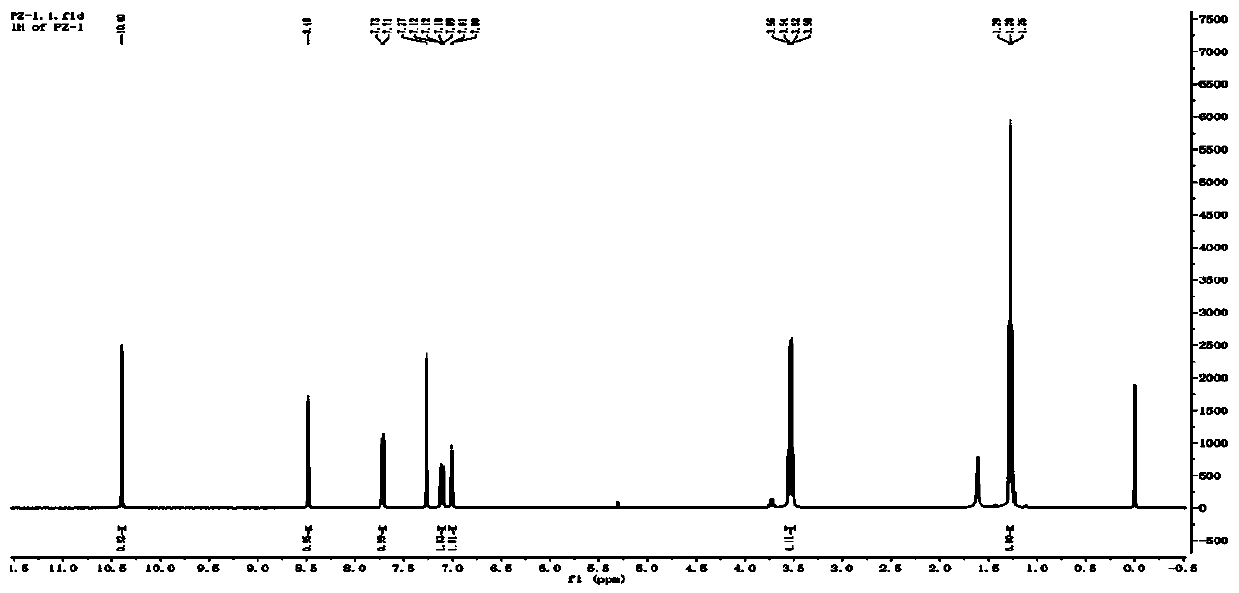
[0050] Add 1.00g (4.8mmol) 2-diethylaminoacetanilide, 0.9g (4.7mmol) DMF to a 50ml two-necked bottle, add 2.5g (8.4mmol) solid phosgene to a 50mL one-necked bottle, add 5mL1, 2-dichloroethane to completely dissolve the solid phosgene, and add the solid phosgene dropwise in an ice-salt bath. The dropwise addition was completed in half an hour. Reaction at 60°C. TLC traced until the reaction was complete. The reaction solution was poured into 50 mL of water, hydrolyzed for 5 min, the organic solvent was spin-dried, filtered with suction, dried, recrystallized from toluene, and dried with suction to obtain 0.82 g of a tan solid. Yield 66%. 1 H NMR (400MHz, Chloroform-d) δ10.40(s,1H),8.48(s,1H),7.72(d,J=9.2Hz,1H),7.11(dd,J=9.2,2.6Hz,1H) , 7.01(d, J=2.5Hz, 1H), 3.53(q, J=7.1Hz, 4H), 1.28(t, J=7.1Hz, 6H).
Embodiment 2
[0052] Preparation of 2-chloro-7-diethylaminoquinoline-3-carbonitrile (2)
[0053] Add 0.30g (1.1mmol) of 3-chloro-7-diethylaminoquinoline-3-carbaldehyde into a one-necked bottle, add 3mL tetrahydrofuran to dissolve, add 0.5g (1.9mmol) of iodine, 7mL ammonia water, stir at room temperature for 3-5h, TLC tracking, until the reaction is completely completed, the reaction solution is poured into water, filtered with suction, and dried. Silica gel column chromatography, eluent: petroleum ether: ethyl acetate (100:0-10, v / v), gave 0.24 g of a yellow solid. Yield: 80%. IR(KBr)cm -1 : 2215(C≡N), 1620(C=N), 688(C-Cl).
Embodiment 3
[0055] Preparation of 2-chloro-7-diethylaminoquinoline-3-carboxylic acid (3)
[0056] Add 0.20g (0.8mmol) 3-chloro-7-ethylenediaminoquinoline-3-carbonitrile into a single-necked bottle, add 2mL of acetic acid, 5mL of 70% sulfuric acid, heat and reflux for 2-3h, TLC traces to the completion of the reaction, Pour the reaction solution into water, filter it with suction, dissolve the obtained solid in 1,2-dichloroethane, add 0.25g (0.84mmol) of solid phosgene, a drop of DMF, reflux for 3-5h, trace by TLC until the reaction is complete, and use a silica gel column Chromatography, eluent: petroleum ether: ethyl acetate, 100:0-50, v / v), eluted to give 0.18g of yellow-green solid, namely the product 2-chloro-7-diethylaminoquinoline-3- Formic acid (3). Yield: 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com