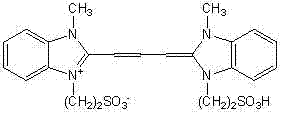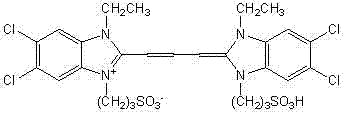Novel pH response fluorescent molecular probe and application thereof
A fluorescent molecular probe and molecular technology, which is applied in the direction of fluorescence/phosphorescence, luminescent materials, material excitation analysis, etc., can solve the problems of inconvenient use, and achieve the effect of simple preparation method and large displacement value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A novel pH-responsive fluorescent molecular probe regulated by intramolecular hydrogen bonds has the following molecular structure:
[0029]
[0030] The preparation method is as follows:
[0031] In a 100 mL three-necked flask equipped with a stirring bar, a condenser, and a thermometer, put 50 mL of ethanol, add a small piece of 0.58 g of metallic sodium, and after the sodium is completely dissolved, add 3.51 g of 1-ethyl-2-methyl- 3-sulfopropyl-5,6-dichlorobenzimidazole betaine and 0.74 g chloral, stirred and heated to reflux for 1 hour. Then cooled to room temperature, filtered, washed with ethanol, and vacuum-dried to obtain the crude product Purified by silica gel column chromatography to obtain purple-red crystals, that is, probe: 1,1′-diethyl-3,3′-disulfopropyl-5,5′,6,6′-tetrachloroimidazocyanine sodium salt.
[0032] The pH-responsive fluorescence performance of the probe was measured in solution by fluorescence method: the fluorescent molecular probe was ...
Embodiment 2
[0034] In this embodiment, the fluorescent molecular probe has the following molecular structural formula:
[0035]
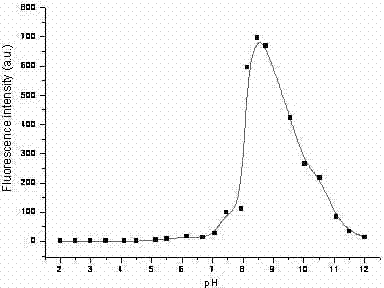
[0036] The pH-responsive fluorescence performance of the probe was measured in solution by fluorescence method: the fluorescent molecular probe was dispersed in dimethyl sulfoxide-water (volume ratio 1:9) at a concentration of 30 μM, and different pH conditions were controlled. Excited with 500 nm light, the fluorescence peak at 605 nm was measured by pH-fluorescence response spectrum. The strong fluorescence region was pH 7.8-8.9, and the fluorescence enhancement factor was 90.
Embodiment 3
[0038] In this embodiment, the fluorescent molecular probe has the following molecular structural formula:
[0039]
[0040] The pH-responsive fluorescence performance of the probe was measured in the solution by the fluorescence method: the fluorescent molecular probe was dispersed in ethanol at a concentration of 20 μM, controlled at different pH conditions, respectively excited by 450 nm light, and the fluorescence at 572 nm The peak was measured by pH-fluorescence response spectrum, the strong fluorescence region was pH 7.7-8.5, and the fluorescence enhancement factor was 82.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com