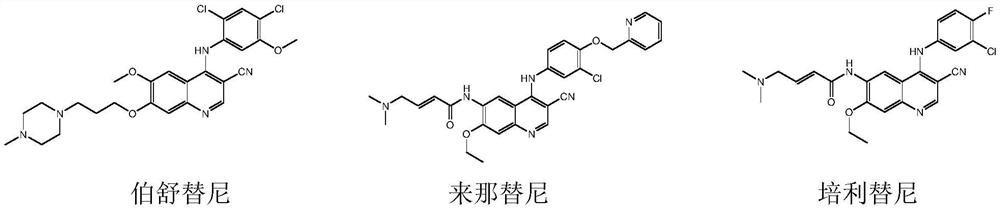
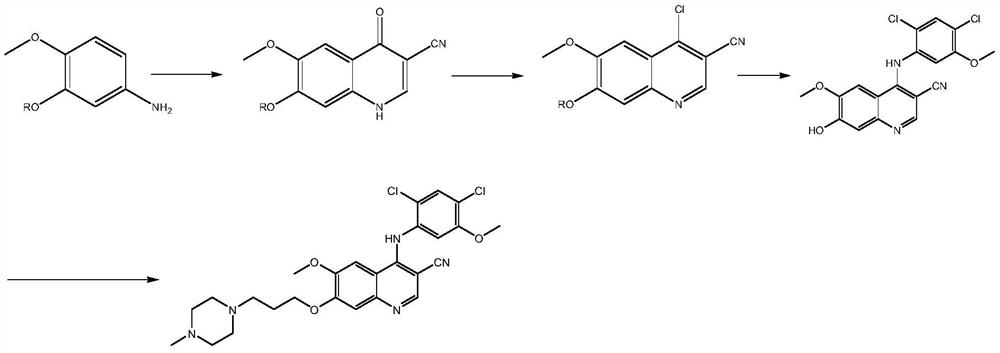
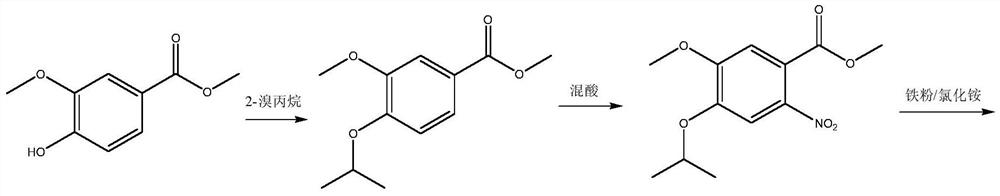
A kind of preparation method of disubstituted 4-chloroquinoline-3-carbonitrile derivative and bosutinib
A technology of bosutinib and chloroquinoline, which is applied in the field of preparation of disubstituted 4-chloroquinoline-3-carbonitrile derivatives and bosutinib, and can solve problems such as high raw material prices, unfavorable industrialization, and large amount of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1: 4-[3-(4-methyl-1-piperazine)]propoxy-5-methoxy-2-nitrobenzoylacetonitrile (III 1 ) preparation
[0088] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser and dropping funnel, add 200 grams of tetrahydrofuran, 36.5 grams (0.1 mol) 4-[3-(4-methyl-1-piperazine)] Methyl propoxy-5-methoxy-2-nitrobenzoate, 6.0 g of acetonitrile, 17.5 g (0.13 mole) of potassium tert-butoxide, stirred at 55 to 60° C. for 3 hours. Cool to 20 to 25°C, use 20wt% ammonium chloride aqueous solution to acidify the pH value of the system to 4.0-4.5, add 200 grams of dichloromethane, separate layers, extract the water layer with dichloromethane 3 times, 50 grams each time, and combine the organic phases , Dichloromethane was reclaimed by distillation to obtain 33.7 grams of 4-[3-(4-methyl-1-piperazine)] propoxy-5-methoxy-2-nitrobenzoyl acetonitrile, and the yield was 89.5 %, the liquid phase purity is 99.4%.
Embodiment 2
[0089] Example 2: 4-[3-(4-methyl-1-piperazine)]propoxy-5-methoxy-2-nitrobenzoylacetonitrile (III 1 ) preparation
[0090] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser and dropping funnel, add 200 grams of tetrahydrofuran, 38.1 grams (0.1 mol) 4-[3-(4-methyl-1-piperazine)] Propoxy-5-methoxy-2-nitrobenzoic acid ethyl ester, 6.0 g of acetonitrile, 10.0 g (0.15 mol) of sodium ethoxide, stirred at 60 to 65° C. for 3 hours. Cool to 20 to 25°C, use 20wt% ammonium chloride aqueous solution to acidify the pH value of the system to 4.0-4.5, add 200 grams of dichloromethane, separate layers, extract the water layer with dichloromethane 3 times, 50 grams each time, and combine the organic phases , Dichloromethane was reclaimed by distillation to obtain 33.3 grams of 4-[3-(4-methyl-1-piperazine)]propoxy-5-methoxy-2-nitrobenzoyl acetonitrile, and the yield was 88.6 %, the liquid phase purity is 99.3%.
Embodiment 3
[0091] Embodiment 3: Formula IV 1 Compound preparation
[0092] In the 500 milliliters of four-neck flasks that are connected with stirring, thermometer, reflux condenser and 20wt% sodium hydroxide aqueous solution absorption device, add 150 grams of DMF, 37.5 grams (0.1 mole) 4-[3- (4-Methyl-1-piperazine)]propoxy-5-methoxy-2-nitrobenzoylacetonitrile (III 1 ), 45.0 grams (0.29 moles) of phosphorus oxychloride, stirred and reacted at 50 to 55° C. for 4 hours. Cool to 20 to 25°C, slowly pour the reaction liquid into 500 grams of water, adjust the pH of the system to 6.0-7.0 with 20 wt% aqueous sodium hydroxide solution, add 200 grams of dichloromethane, separate layers, and extract the water layer with dichloromethane 3 times, 50 grams each time, the organic phases were combined, and dichloromethane was reclaimed by distillation to obtain 35.3 grams of formula IV 1 Compound, the yield is 83.6%, and the liquid phase purity is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com