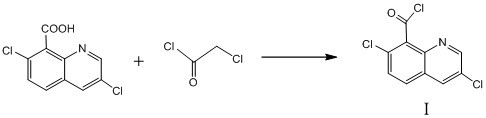
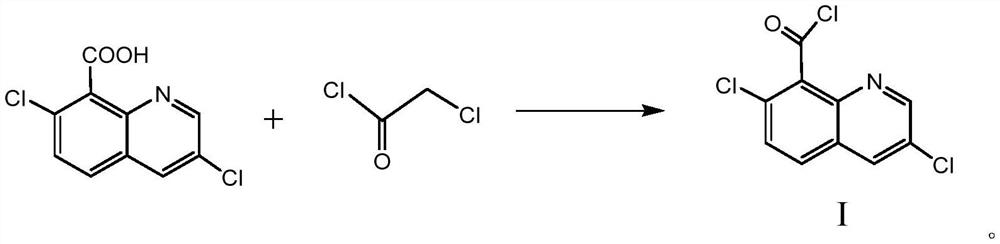
A kind of synthetic method of 3,7-dichloro-8-quinoline formyl chloride
A synthetic method, quinolineformyl technology, applied in the field of artificial antigen synthesis, can solve the problems of increasing the difficulty of "three wastes" and "three wastes" treatment, unfavorable safety and environmental protection, and high energy consumption of various solvents, achieving low cost, The effect of good product quality and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Synthesis of 3,7-dichloro-8-quinoline formyl chloride
[0027] (1) In a 250ml four-neck flask equipped with a magnetic stirrer, a thermometer and a lead tube condenser, add 24.2g3,7-dichloro-8-quinolinic acid and 45.2g monochloroacetyl chloride, and in the process of stirring 0.12 g of catalyst anhydrous aluminum trichloride was added, the condenser was turned on to lower the temperature, and the temperature was slowly raised to 100°C-115°C, and the reaction was refluxed at this temperature for 1.5h.
[0028] (2) Sampling control, when the content of 3,7-dichloro-8-quinolinic acid is ≤0.5%, the reaction end point is reached, the temperature is lowered to 50°C, filtered, and the filtrate is collected for later use.
[0029] (3) the filter cake is washed twice with chloroacetyl chloride, each washing with 10g of chloroacetyl chloride, the combined washing liquid and the filtrate collected in step (2), then underpressure distillation, and then after 95 ℃ of under...
Embodiment 2
[0030] Example 2 Synthesis of 3,7-dichloro-8-quinoline formyl chloride
[0031] With step (1), middle catalyst aluminum trichloride is replaced by anhydrous iron trichloride, other is identical with embodiment 1, obtains pale yellow 3,7-dichloro-8-quinoline formyl chloride 25.3g, yield It is 94.3% and the purity is 97.1%.
Embodiment 3
[0032] Example 3 Synthesis of 3,7-dichloro-8-quinoline formyl chloride
[0033] In step (1), the amount of monochloroacetyl chloride was modified to 33.9g, and the others were the same as in Example 1 to obtain 25.7g of pale yellow 3,7-dichloro-8-quinoline carboxylic acid chloride, with a yield of 96.48% , with a purity of 97.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com