Synthesis method of quizalofop-p-ethyl
A technology of quizalofop-p-p and dichloroquinoxaline, which is applied in the field of synthesis of the herbicide quizalofop-p-p-p-p in rice fields, can solve problems such as low efficiency, low optical purity, and large pollution, so as to increase reaction yield and improve optical efficiency. Purity and the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
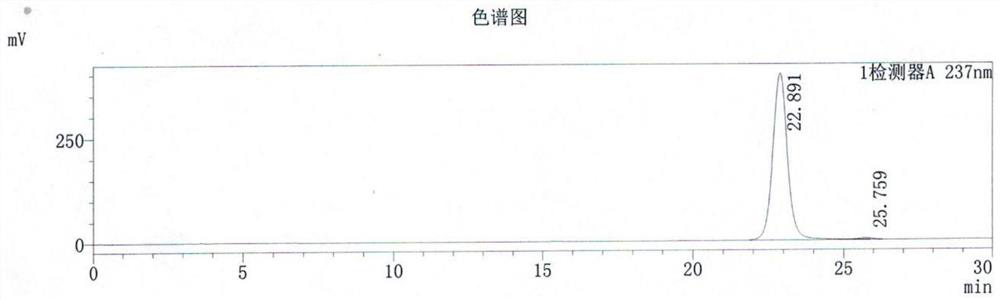
Embodiment 1
[0041]199.0KG (1.0kmol) 2,6-dichloroquinoxaline, 210.2KG (1.0kmol, optical purity 99.9%) R (+) 2-(4-hydroxyphenoxy) ethyl propionate, 207.3KG (1.5 kmol) potassium carbonate, 32.2KG (0.1kmol) catalyst tetrabutylammonium bromide, 460.0KG toluene into the reactor, stirring at room temperature, heating up to 70-80 ° C for about 3-4 hours, while reacting at -0.07MPa Under the pressure of ~-0.08MPa, depressurize and reflux to separate water, and then react with nitrogen gas at the bottom of the kettle for about 3-4 hours, and take samples for inspection until the content of 2,6-dichloroquinoxaline is ≤0.5%, and the reaction is terminated.
[0042] After the reaction is finished, cool down to 40-50°C within 30 minutes, wash the reaction solution with water several times until the water layer is absolutely neutral, separate layers, decolorize and dissolve the organic layer, crystallize with absolute ethanol, filter, and dry to obtain 364.4KG product.
[0043] The total reaction time ...
Embodiment 2
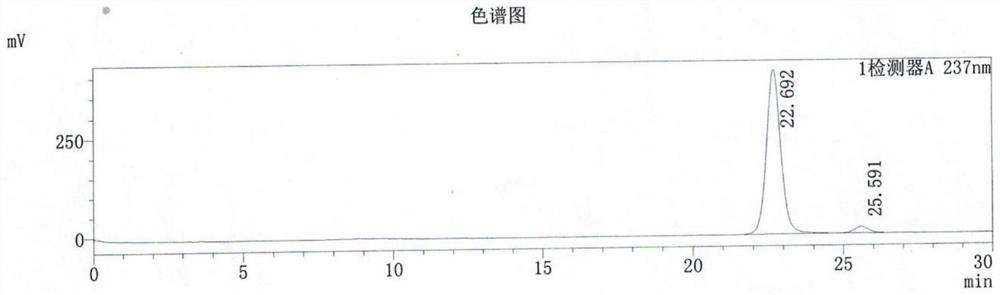
[0044] Embodiment 2: Except that the reaction temperature is replaced by 65-70° C., all the others are the same as in Embodiment 1, and 361.0 KG of the product are obtained.
[0045] The total reaction time of this example is about 8-10 hours, the chemical content of quizalofop-p-ethyl is 97.7%, the yield is 94.61%, and the optical purity is >99%.
Embodiment 3
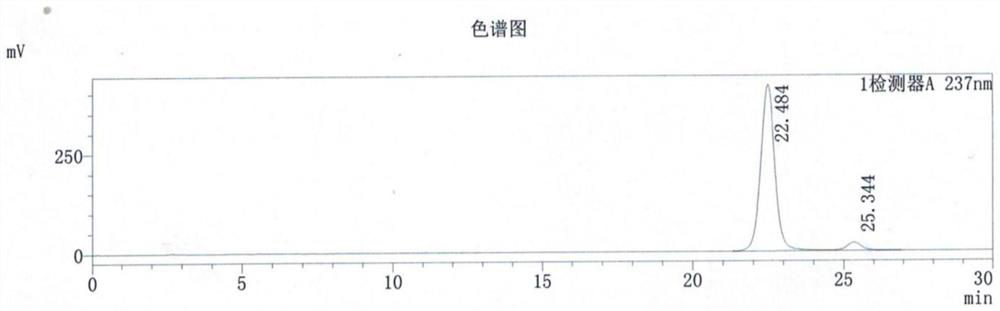
[0046] Embodiment 3: Except that the reaction temperature is replaced by 80-85° C., all the others are the same as in Embodiment 1, and 362.2 KG of the product are obtained.
[0047] The total reaction time of this example is about 5-6 hours, the chemical content of quizalofop-p-ethyl is 97.6%, the yield is 94.82%, and the optical purity is >99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com