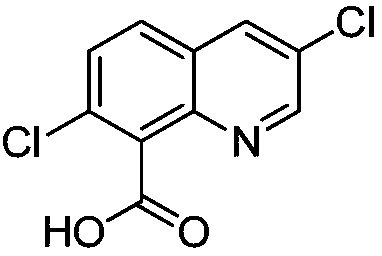
Preparation method of quinclorac
A technology of quinclorac and quinoline carboxylic acid, applied in the field of herbicides, can solve the problems of unfavorable cleaning process production, inability to recycle and apply, and high cost of three wastes treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a kind of preparation method of quinclorac, wherein, the method comprises the following steps,
[0025] 1) Using N-hydroxyphthalimide compounds and azobisisobutyronitrile as catalysts and oxygen as an oxidant to oxidize 7-chloro-8-methylquinoline to obtain 7-chloro-8-quinoline morphine carboxylic acid;
[0026] 2) Using azobisisobutyronitrile as a catalyst, chlorinating 7-chloro-8-quinolinecarboxylic acid and chlorine to obtain 3,7-dichloro-8-quinolinecarboxylic acid.
[0027] According to the method of the present invention, by using N-hydroxyphthalimide compounds and azobisisobutyronitrile as a catalyst, oxygen is used as an oxygenant to oxidize 7-chloro-8-methylquinoline, which can The generation of waste acid and waste water in the oxidation process can be avoided, and 7-chloro-8-quinolinecarboxylic acid can be obtained with low reaction temperature, simple post-treatment and high yield.
[0028] In step 1), the amount of the N-hydroxyphthal...
Embodiment 1
[0043] 1) In reactor A, add 200 grams of ice water, slowly add 600 grams of concentrated sulfuric acid, then add 423 grams of 3-chloro-2-methylaniline, 1 gram of sodium iodide, and slowly add 290 grams of glycerin at 120°C , The dropwise addition was completed in 4 hours, and the temperature was kept at 140° C. for 3 hours. After the reaction finishes, cool down to 30°C, add 200 grams of water, 1200 grams of sherwood oil (boiling range 90-120°C), adjust the pH to 9~10 with 20% by weight of sodium hydroxide solution, then heat up to 80°C for 1 Hours, static layering, camera precipitated until no fraction was formed to obtain 497.7 g of 7-chloro-8-methylquinoline with a content of 98.3% by weight and a yield of 92.1%.
[0044] 1 H-NMR(500MHz,d6-DMSO):δ8.991-8.98(dd,1H,J 1 =1.5Hz,J 2 =1.5Hz), 8.405-8.386(dd,1H,J 1 =1.5Hz,J 2 =1.5Hz)7.874-7.857(d,1H,J=8.5Hz),7.64-7.622(d,1H,J=9Hz),7.596-7.572(dd,1H,J 1 =4Hz,J 2 = 4Hz), 2.799(s, 3H). LCMS (M+1): 178.1.
[0045] 2) In react...
Embodiment 2
[0050] 1) In reactor A, add 200 grams of ice water, slowly add 600 grams of concentrated sulfuric acid, then add 423 grams of 3-chloro-2-methylaniline, 1 gram of sodium iodide, and slowly add 290 grams of glycerin at 120°C , The dropwise addition was completed in 4 hours, and the temperature was kept at 140° C. for 3 hours. After the reaction finishes, cool down to 30°C, add 200 grams of water, 1200 grams of sherwood oil (boiling range 90-120°C), adjust the pH to 9~10 with 20% by weight of sodium hydroxide solution, then heat up to 80°C for 1 Hours, static layering, camera precipitation until no fraction is generated, 497.7 grams of 7-chloro-8 methylquinoline (confirmed by nuclear magnetic and mass spectrometry data to obtain 7-chloro-8 methylquinoline), content 98.3% by weight , yield 92.1%.
[0051] 2) In reactor B, add 300 grams of 7-chloro-8-methylquinoline, 300 milliliters of acetonitrile solution, 2.76 grams of N-hydroxyphthalimide, and 4.17 grams of azobisisobutyronitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com