Hydroxychloroquine sulfate crystal form B and preparation method thereof
A technology of hydroxychloroquine sulfate crystals and hydroxychloroquine, applied in organic chemical methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of poor hygroscopicity, storage stability and low solubility, and achieve hygroscopicity Small size, improved physical and chemical properties, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 hydroxychloroquine sulfate crystal form B
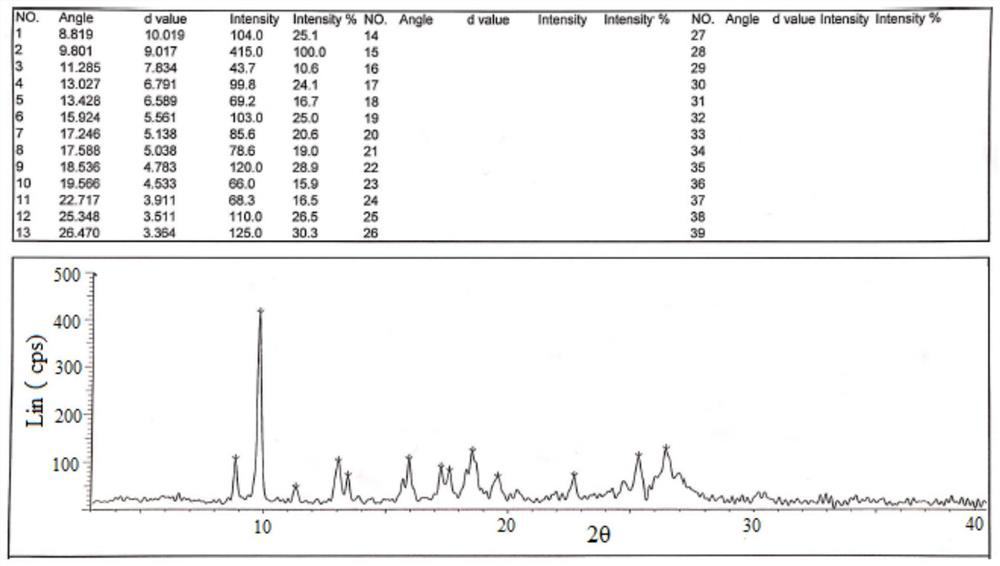
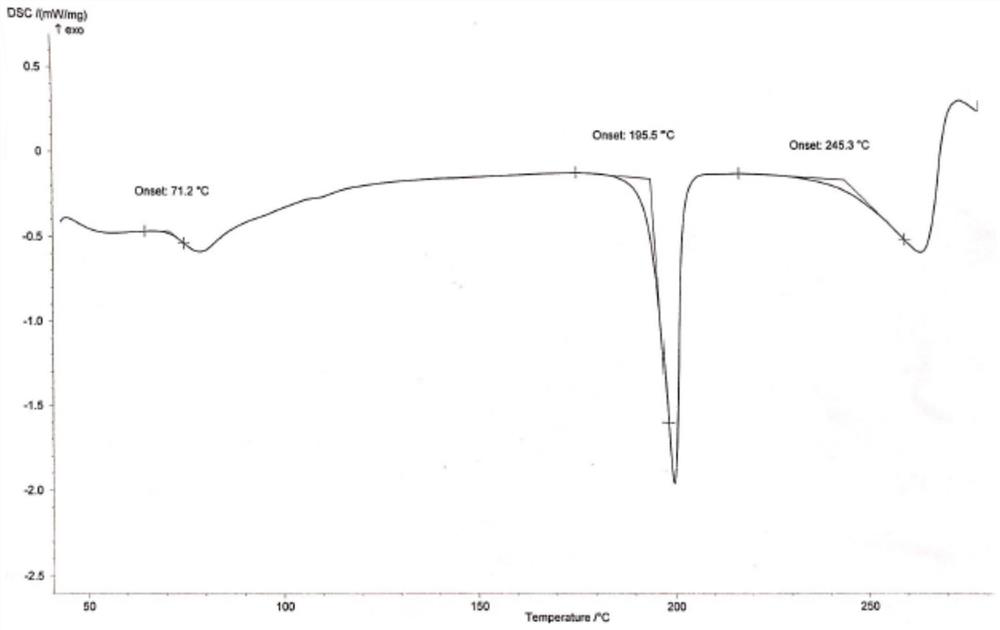
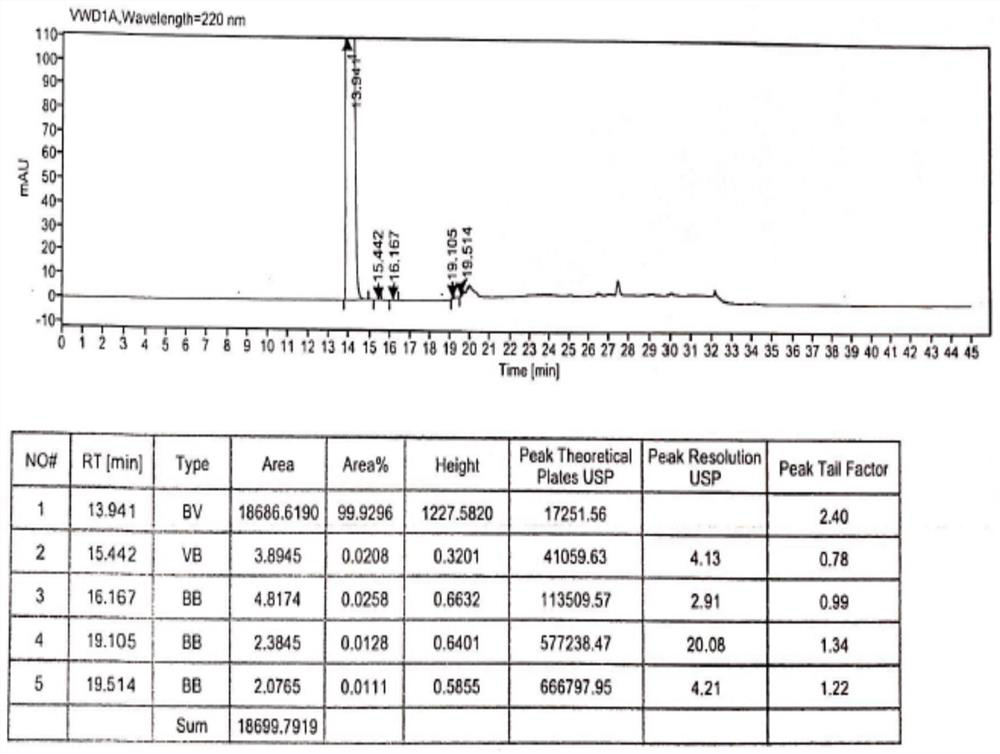
[0036] Add 1100g of hydroxychloroquine and 8800ml of ethanol into the reaction flask, stir until dissolved, and control the temperature at 5-10°C. 330 g of 70% sulfuric acid aqueous solution was added dropwise, and the temperature was controlled not to exceed 10°C. After the dropwise addition, keep stirring at 5-10° C. for 12 h, filter, and vacuum-dry at 50° C. to constant weight to obtain 1401.2 g of hydroxychloroquine sulfate in crystal form B, with a yield of 98.6% and an HPLC purity of 99.93% (see figure 1 , XRD pattern see figure 2 , HPLC spectrum see image 3 ).
[0037] The preparation of comparative example 1 hydroxychloroquine sulfate A crystal form
[0038] Add 1100g of hydroxychloroquine and 880ml of ethanol into the reaction flask, stir until dissolved, and control the temperature at 35-40°C. Add 330 g of sulfuric acid aqueous solution with a mass fraction of 70% dropwise, and ...
Embodiment 2
[0041] Embodiment 2 investigates the test of the stability of hydroxychloroquine sulfate crystal form
[0042] 1. Influencing factor experiment
[0043] Carry out the influence factor test of this product and the crystal form in comparative example 1 and comparative example 2 according to (Chinese Pharmacopoeia 2015 edition two appendix XIX C) relevant regulations.
[0044] (1) High temperature test: take a proper amount of the test product and place it in a temperature-adjusting and humidity-adjusting box, and place it at 60°C for 60 days, and take samples on the 5th, 10th, 30th and 60th day, and focus on the investigation according to the stability The project requires testing.
[0045] Table 1 High temperature test results
[0046]
[0047] (2) High humidity test: take a proper amount of the test product and place it in a temperature-adjusting and humidity-adjusting box, and place it at 25°C under the condition of relative humidity of 90%±5% for 5 days, 10 days, 30 day...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com