Inhalant containing chloroquine therapeutic agent and preparation method of inhalant
A technology for a therapeutic agent and an inhalant, which is applied to the field of inhalants containing chloroquine therapeutic agents and the preparation thereof, can solve the problems of heavy reaction, low utilization rate, long course of treatment, etc., and achieves enhanced stability and improved use efficiency. , strong targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
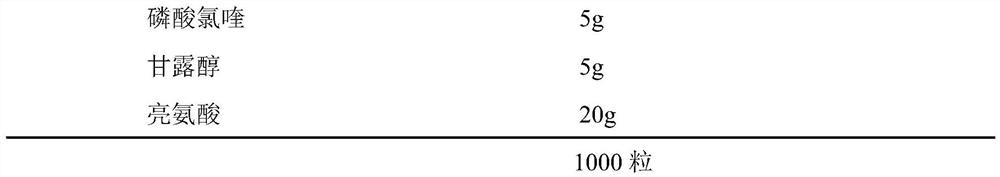
[0039] A kind of chloroquine phosphate powder spray for inhalation, its prescription consists of
[0040]
[0041] Preparation Process:
[0042] (1) dissolving leucine in water for injection, adding chloroquine phosphate and mannitol, stirring to form a uniform solution;
[0043] (2) adopt spray drying technology to carry out spray drying to above-mentioned solution, obtain chloroquine phosphate inhalation powder spray;
[0044] The spray drying process conditions are: air inlet temperature: 118°C±2°C; material temperature: 60°C, atomization pressure: 170kPa, liquid feed rate: 12.0mL / min, solid-liquid content of the mixture: 15mg / mL.
[0045] (3) Fill the dried chloroquine phosphate dry powder in 3#HPMC vegetable capsules, and then seal them with a capsule encapsulation machine.
Embodiment 2
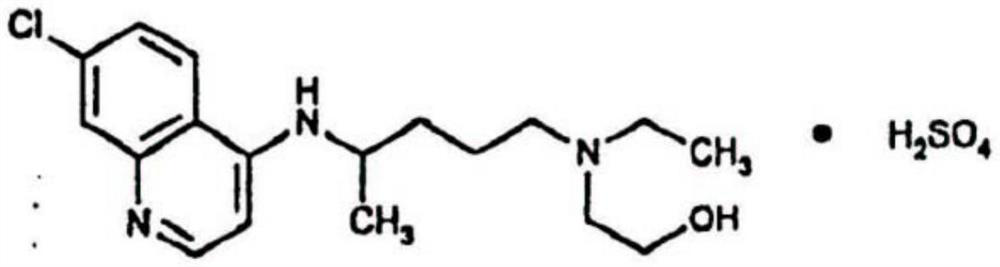
[0047] A kind of hydroxychloroquine sulfate powder spray for inhalation, its prescription consists of
[0048]
[0049]
[0050] Preparation Process:
[0051] (1) dissolving leucine in water for injection, adding hydroxychloroquine sulfate and mannitol, stirring to form a uniform solution;
[0052] (2) adopt spray drying technology to carry out spray drying to above-mentioned solution, obtain hydroxychloroquine sulfate powder mist inhalation;
[0053] The spray drying process conditions are: air inlet temperature: 118°C±2°C; material temperature: 60°C, atomization pressure: 170kPa, liquid feed rate: 12.0mL / min, solid-liquid content of the mixture: 15mg / mL.
[0054] (3) Fill the dried hydroxychloroquine sulfate dry powder in 3#HPMC vegetable capsules, and then seal them with a capsule encapsulation machine.
Embodiment 3
[0056] A kind of hydroxychloroquine sulfate powder spray for inhalation, its prescription consists of
[0057]
[0058] Preparation Process:
[0059] (1) dissolving leucine in water for injection, adding hydroxychloroquine sulfate and lactose, stirring to form a uniform solution;
[0060] (2) adopt spray drying technology to carry out spray drying to above-mentioned solution, obtain hydroxychloroquine sulfate powder mist inhalation;
[0061] The spray drying process conditions are: air inlet temperature: 118°C±2°C; material temperature: 60°C, atomization pressure: 170kPa, liquid feed rate: 12.0mL / min, solid-liquid content of the mixture: 15mg / mL.
[0062] (3) Fill the dried hydroxychloroquine sulfate dry powder in 3#HPMC vegetable capsules, and then seal them with a capsule encapsulation machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com