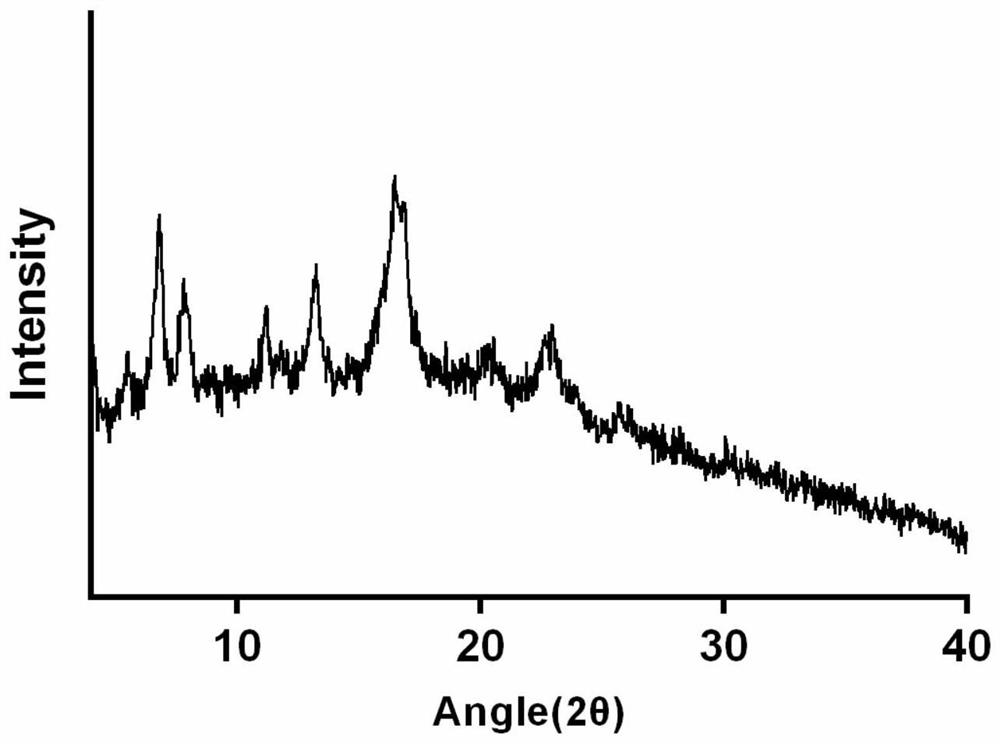
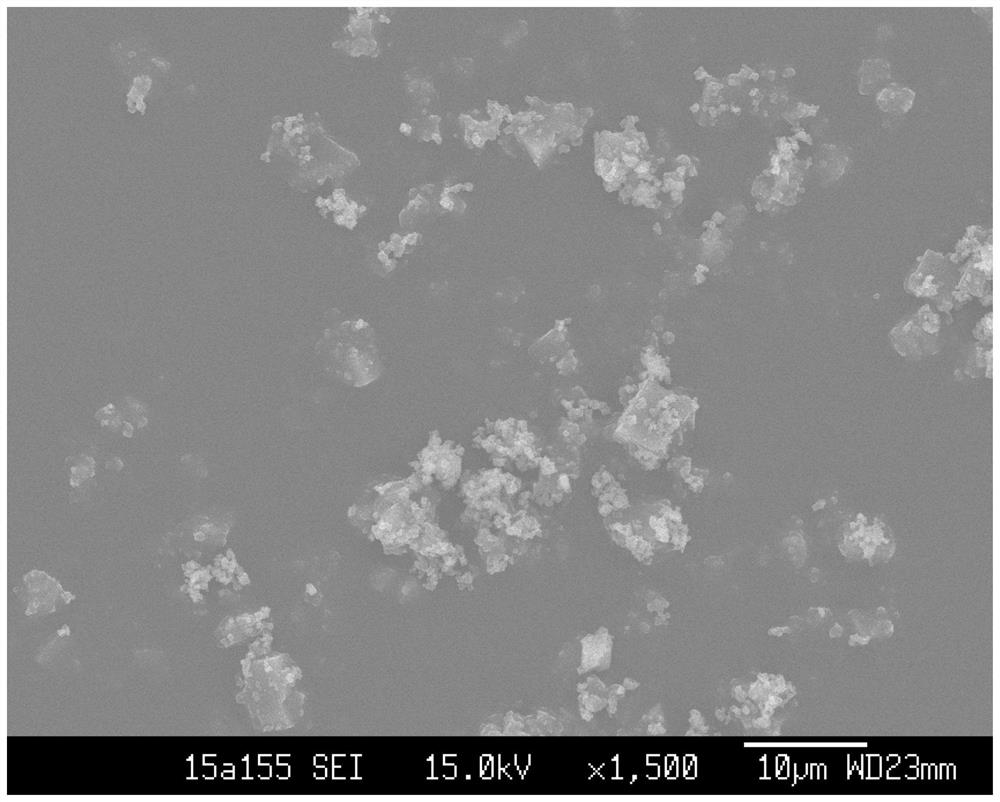
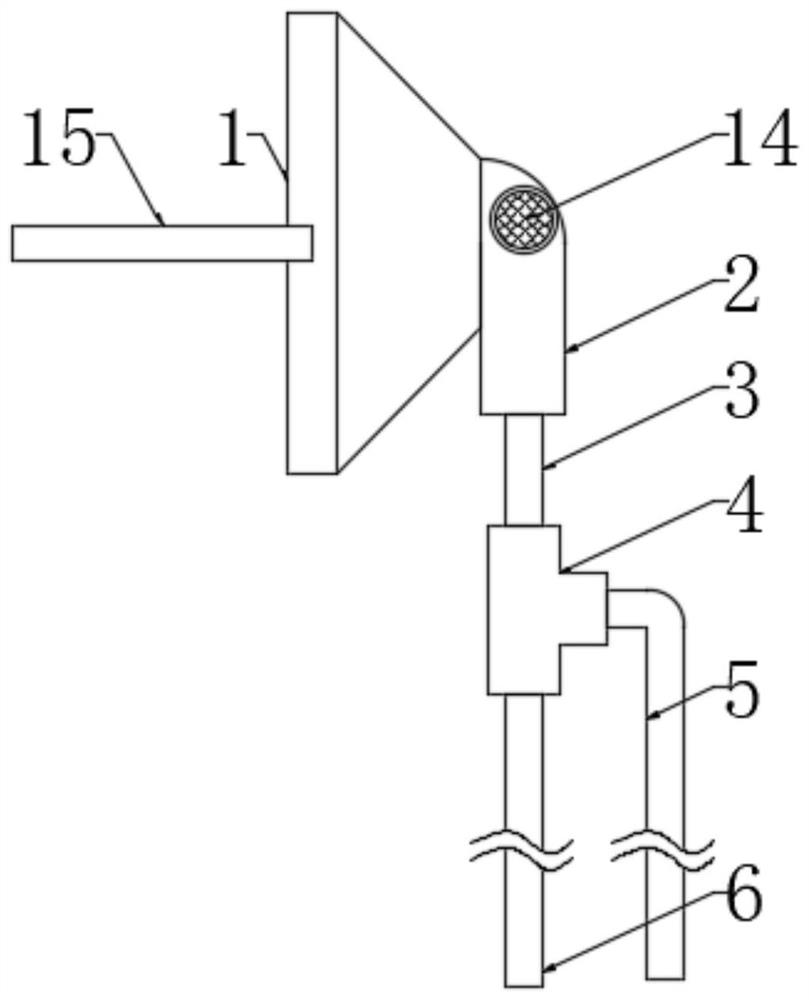
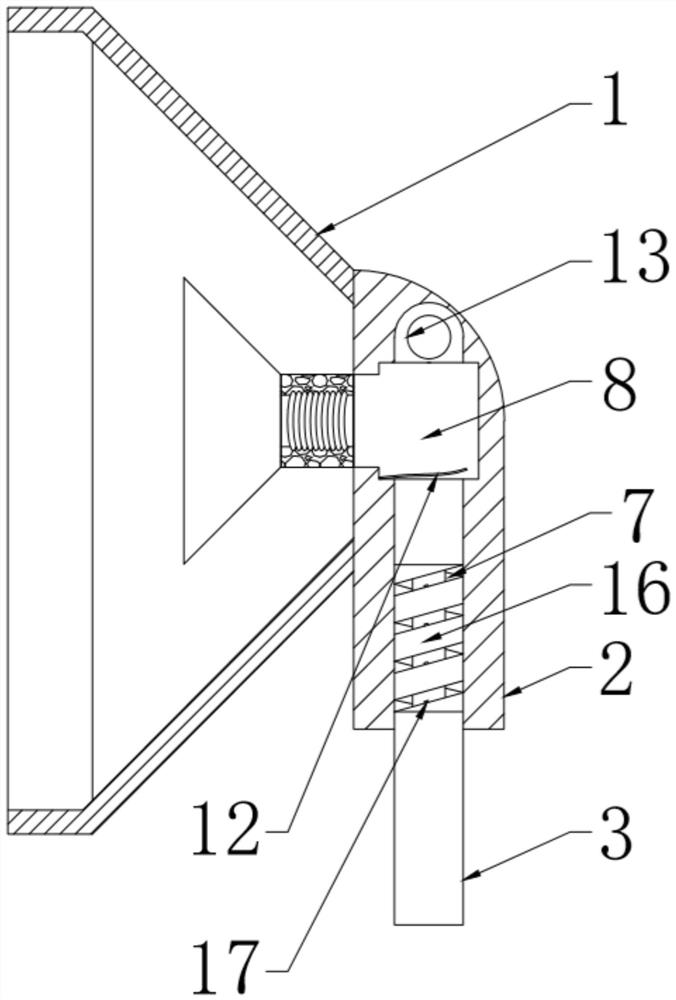
Patents
Literature
64 results about "Inhalant Solution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
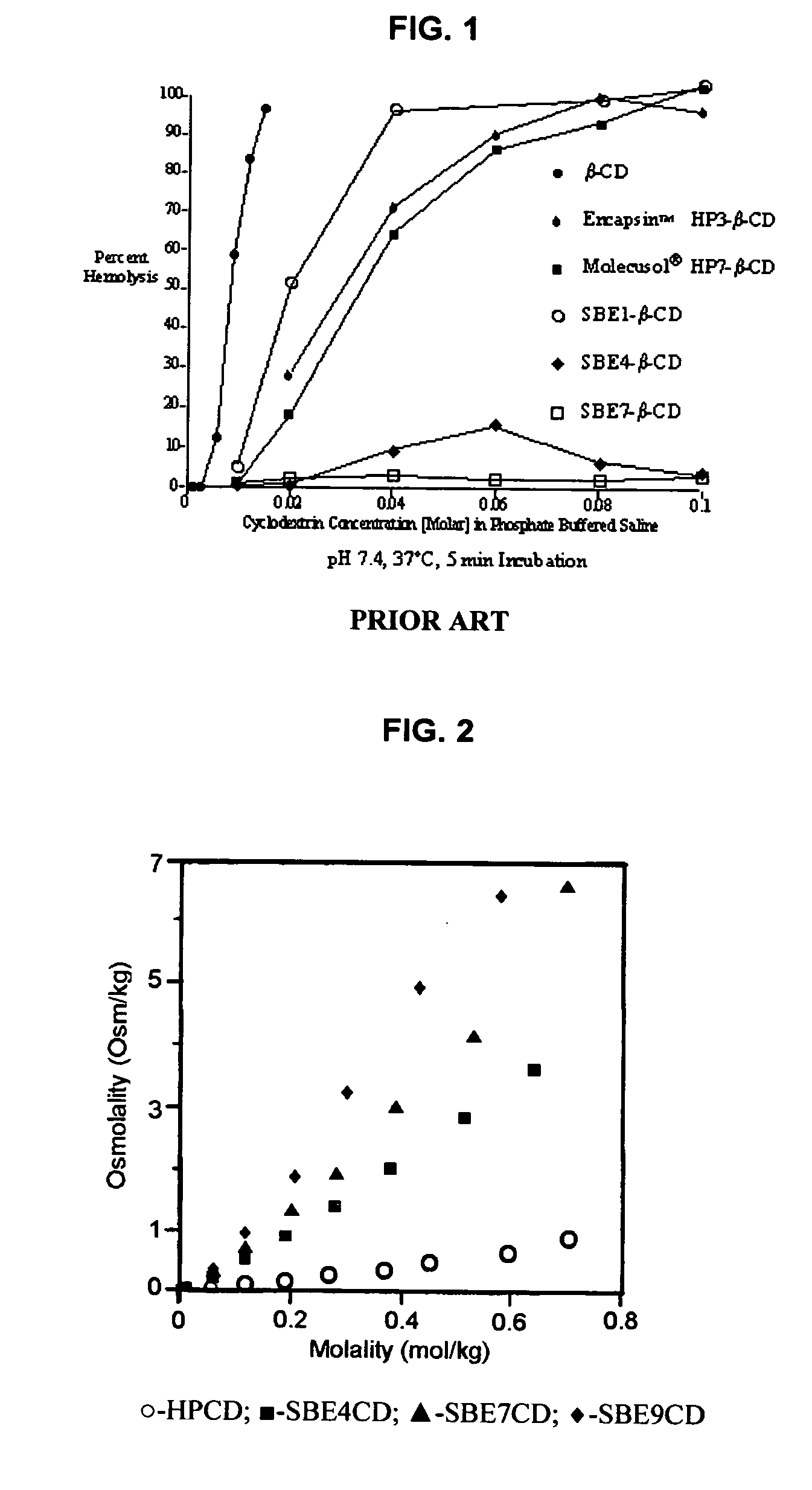
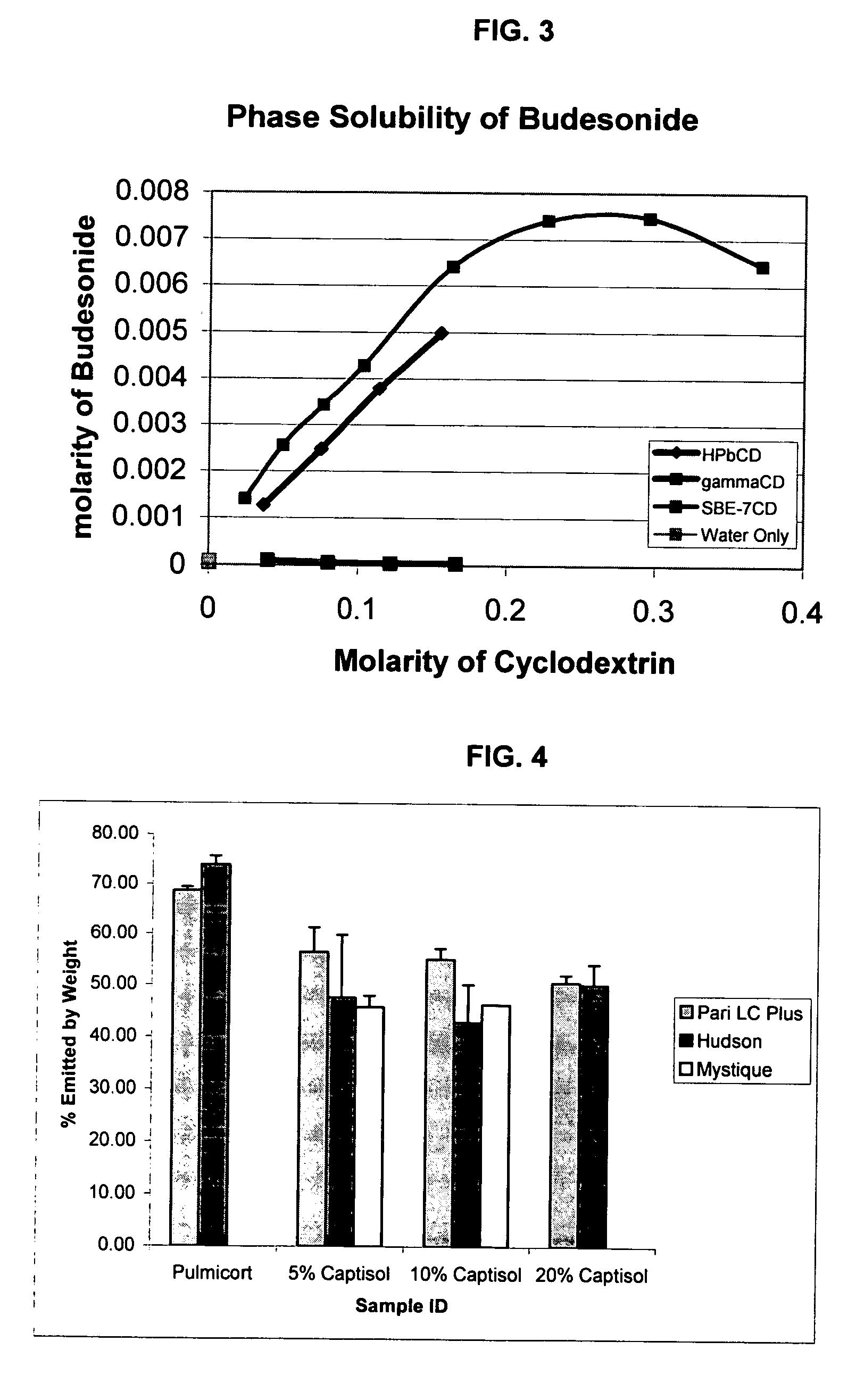
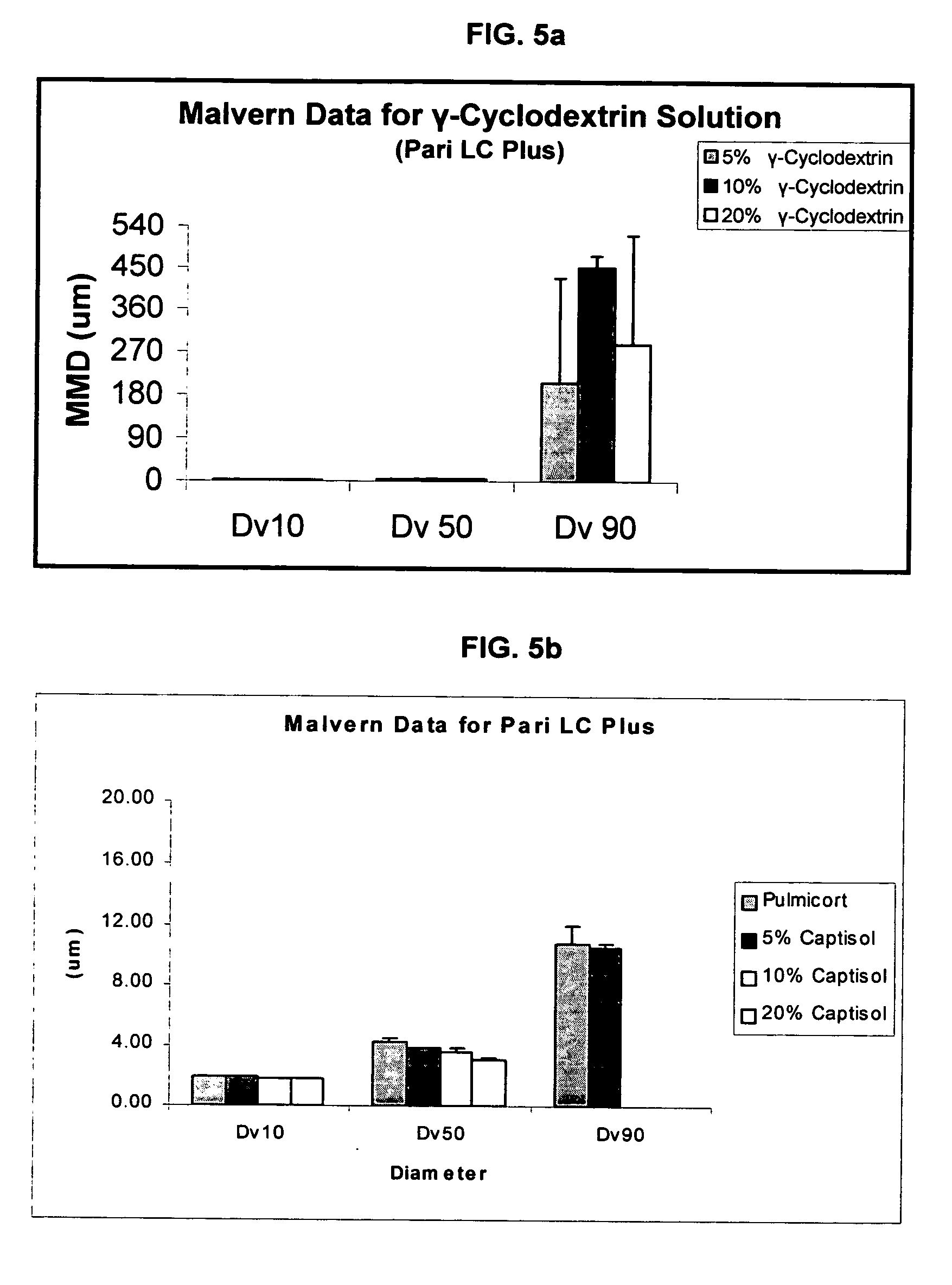
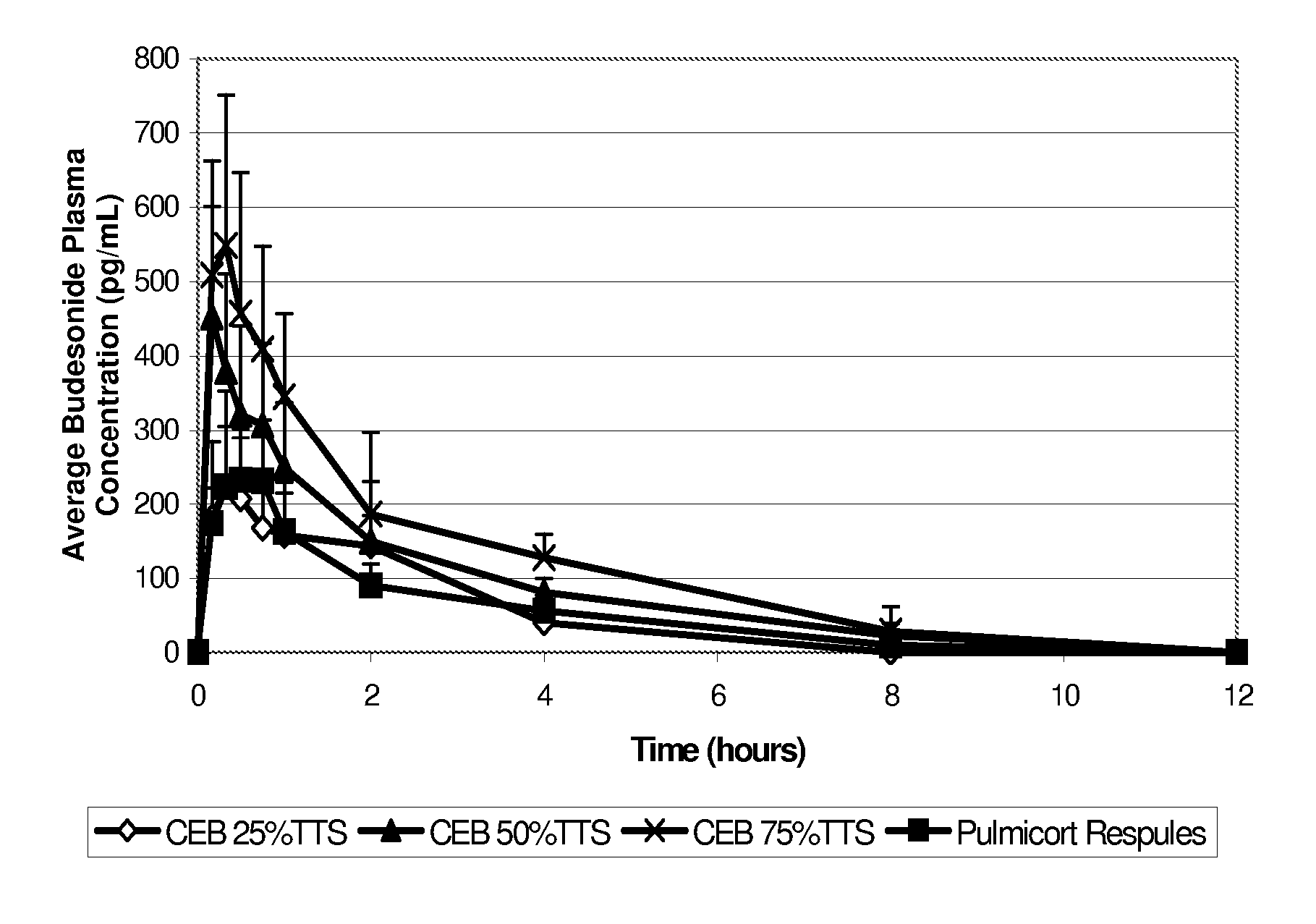
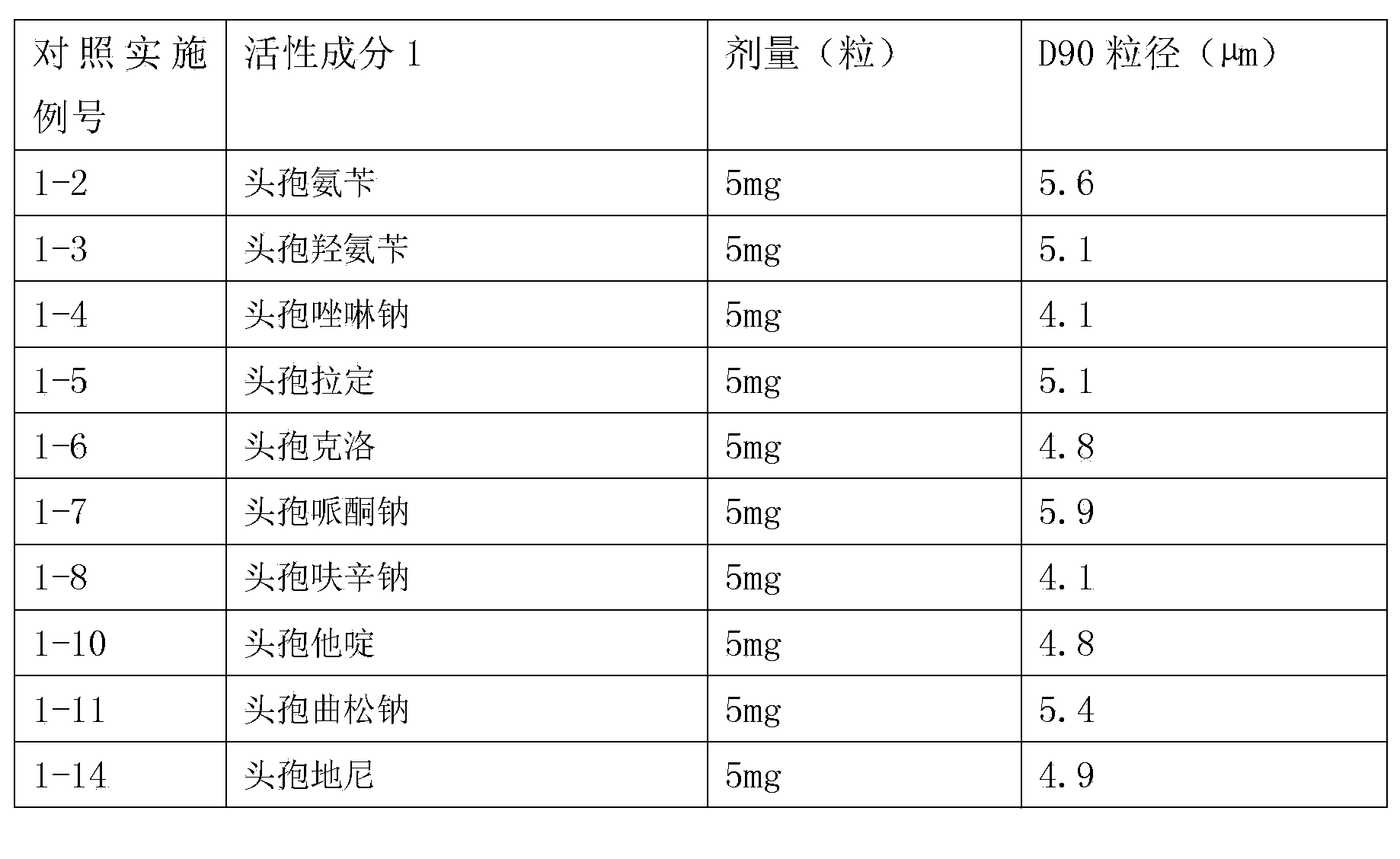
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
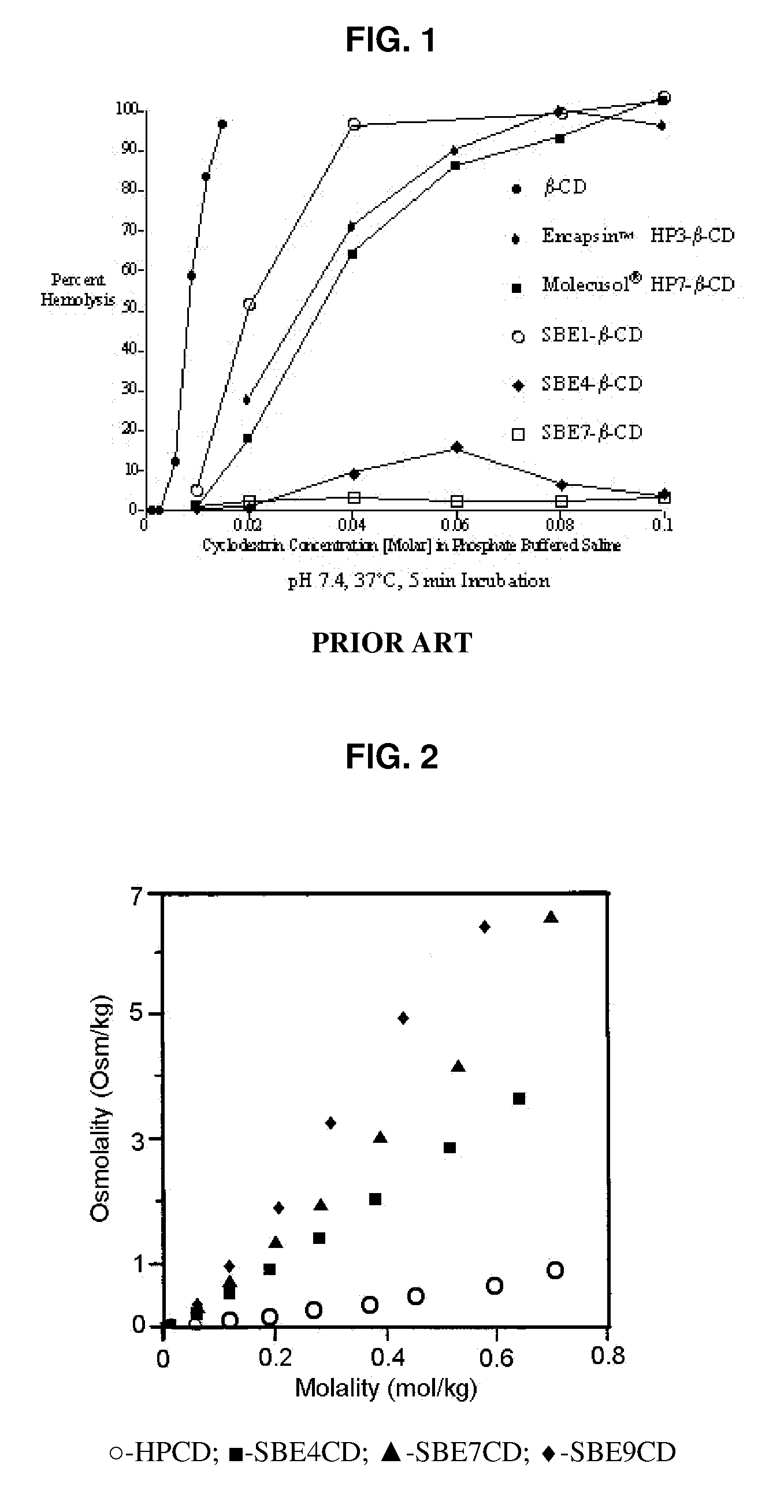
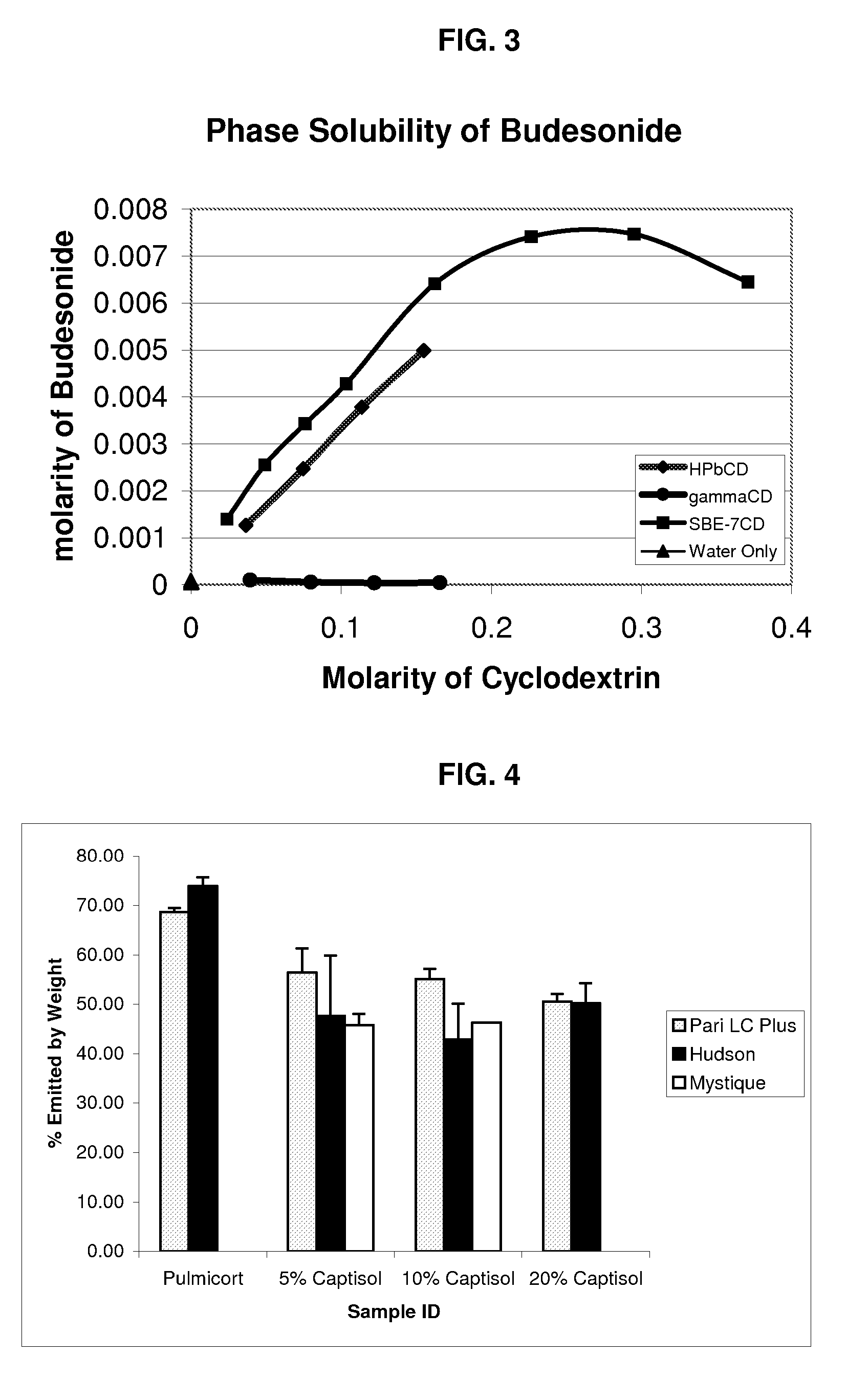
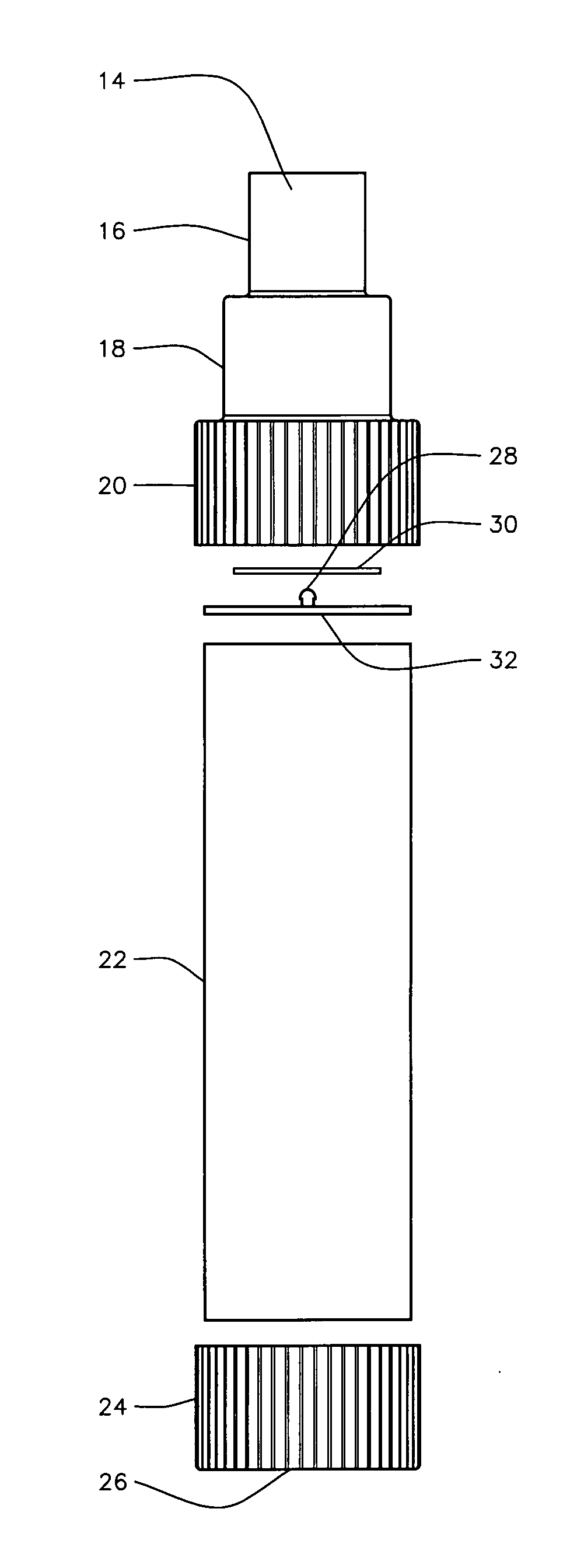
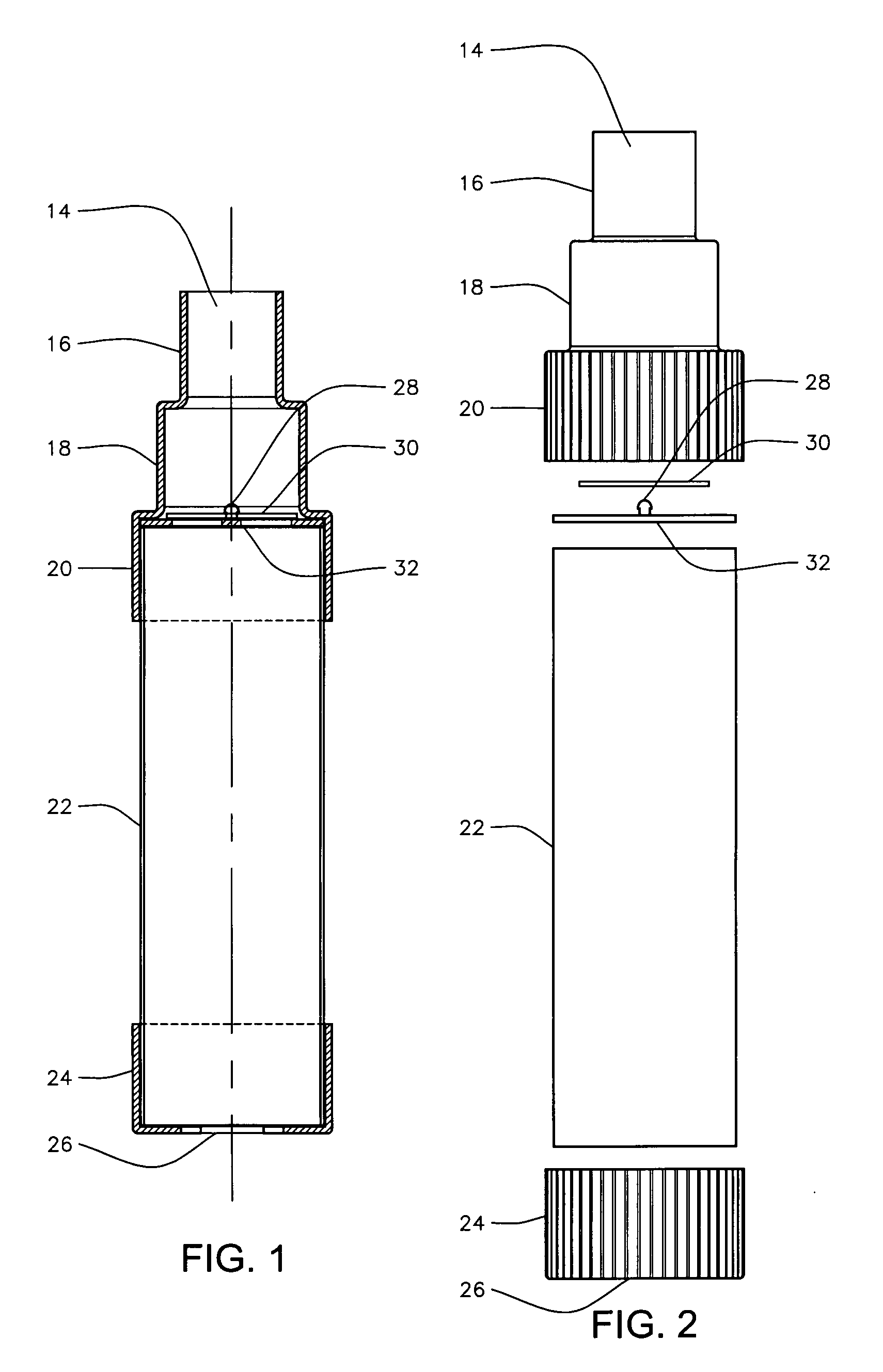
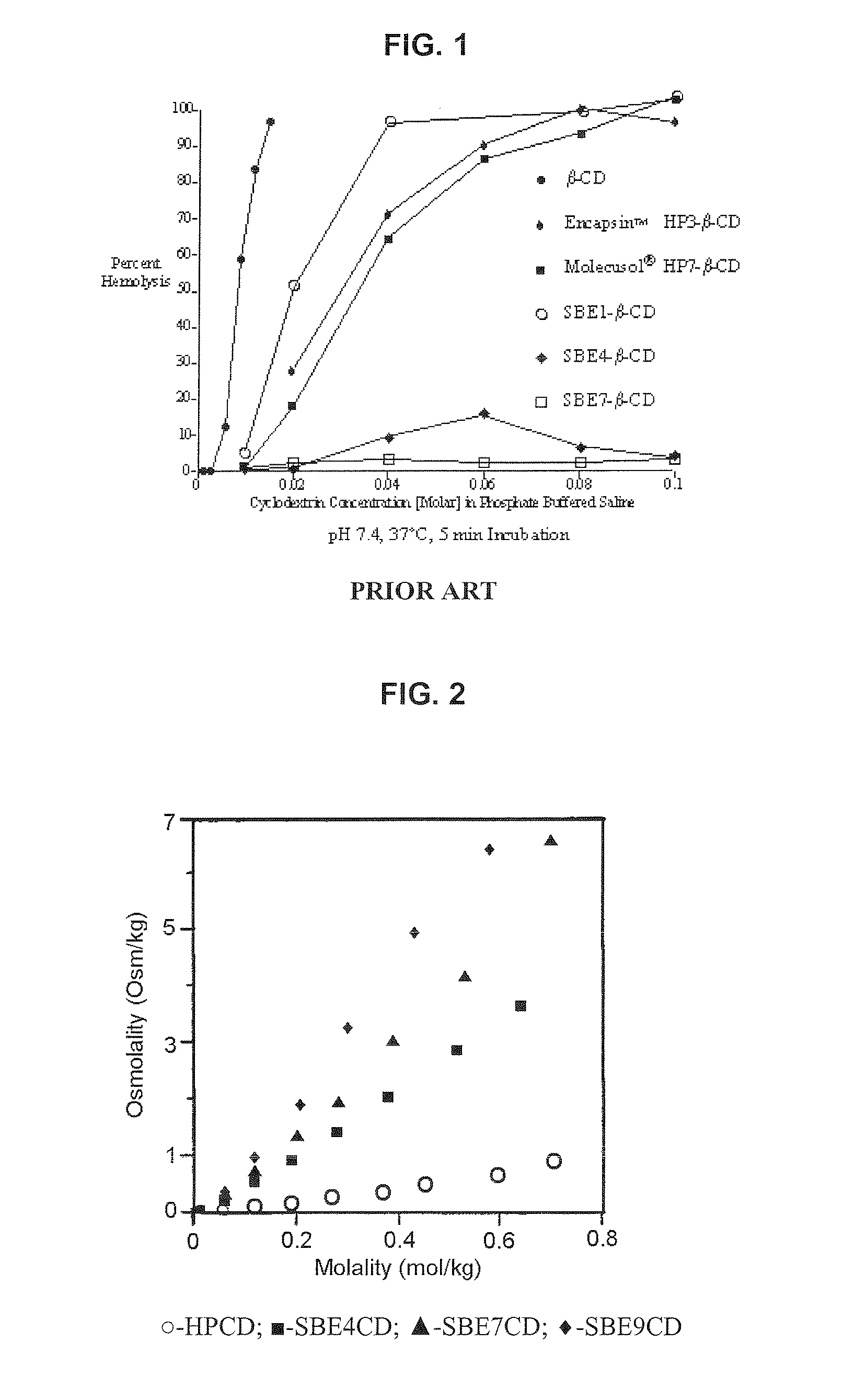
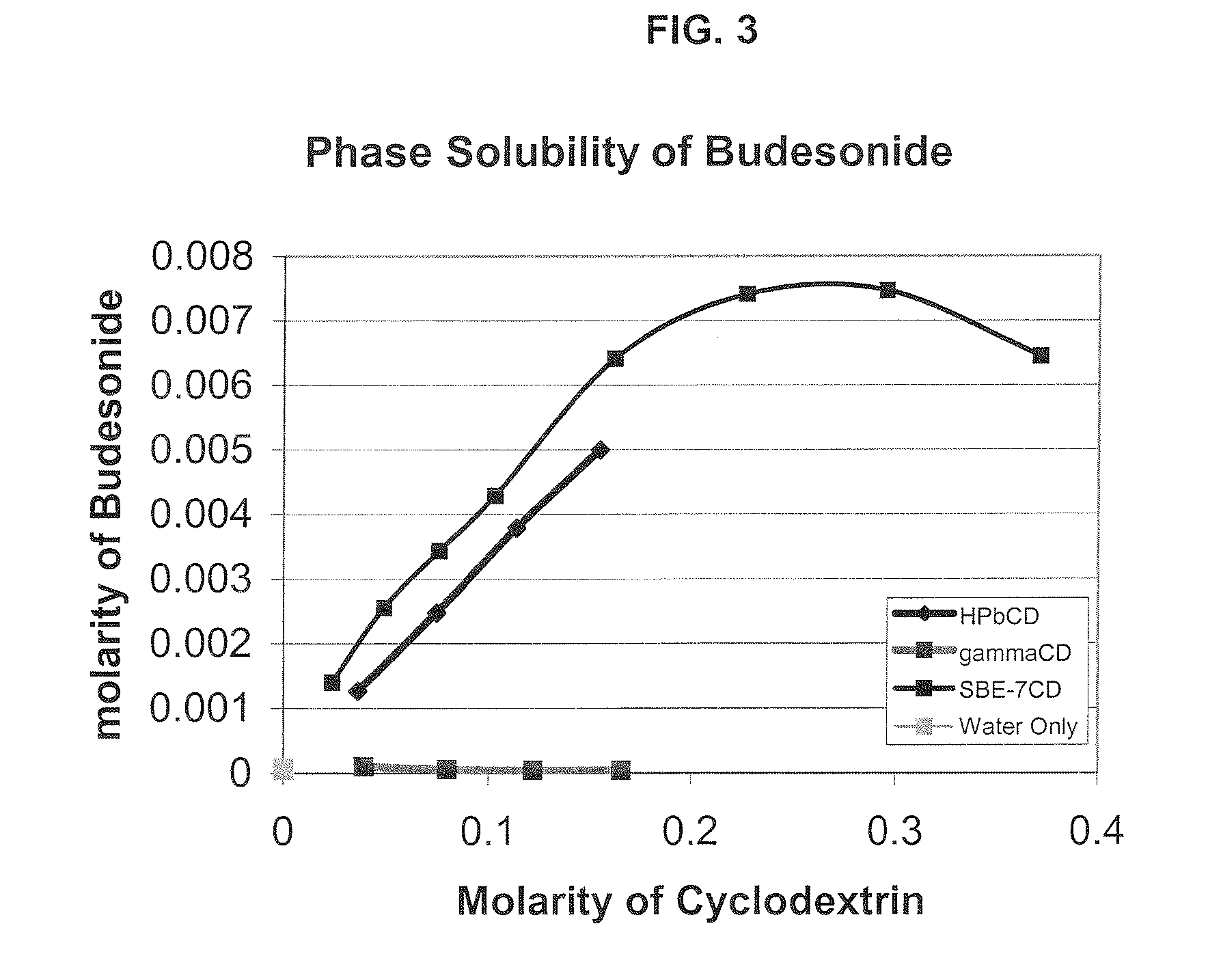
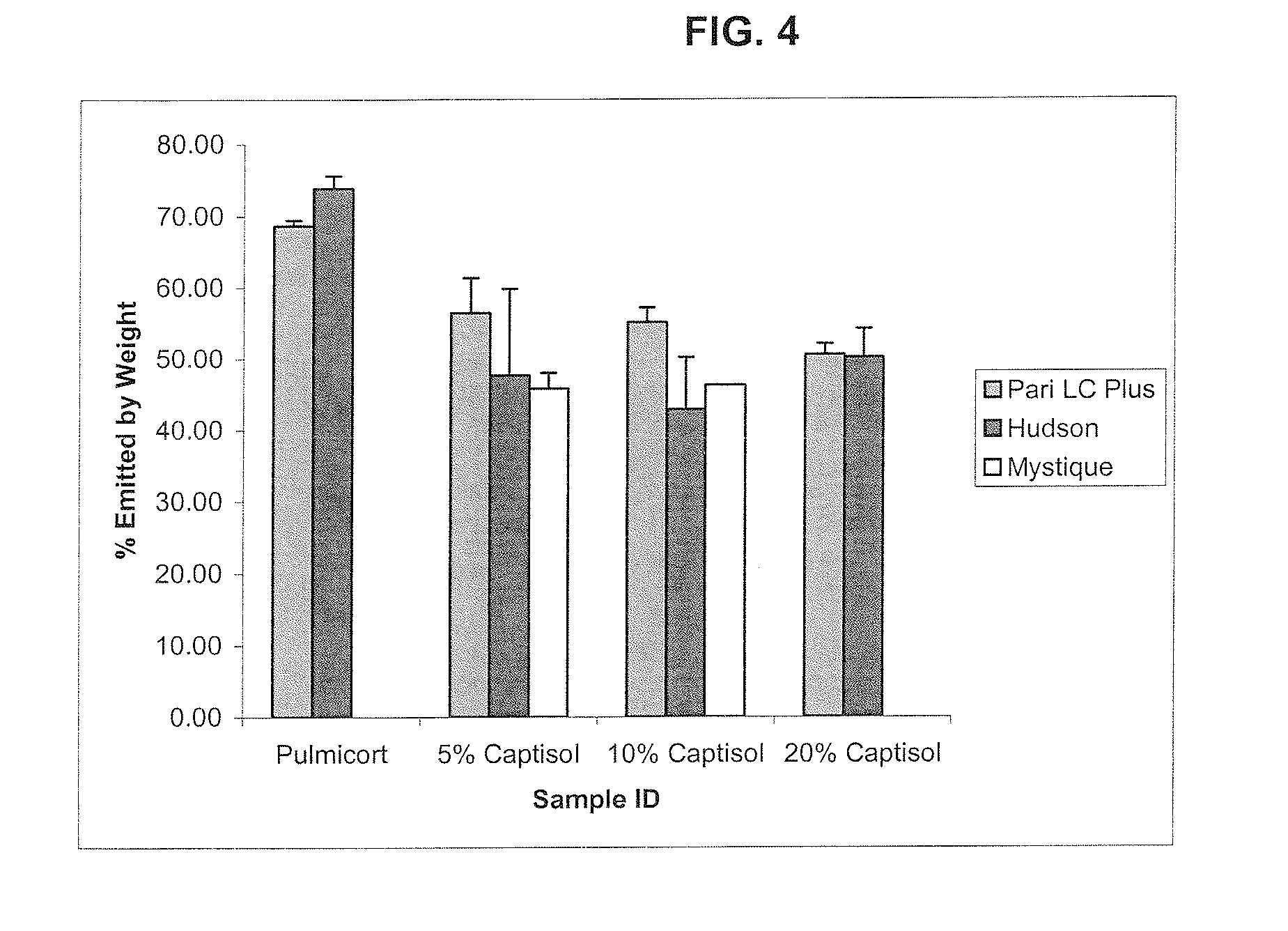
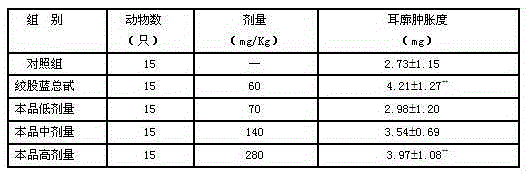
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
Inhalant Formulation Containing Sulfoalkyl Ether Cyclodextrin and Corticosteroid
InactiveUS20070202054A1Improve solubilityImprove stabilityBiocidePowder deliveryNebulizerCyclodextrin
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMA INC
Disposable antistatic spacer
InactiveUS20080210225A1Readily availableAffordable to recycleMedical devicesMedical atomisersTherapeutic DevicesAntibiotic Y
A “disposable antistatic spacer” is presented for use as a universal medicinal inhalant applicator offering an inexpensive tube configuration with antibacterial and biodegradable characteristics for efficient administration of pharmaceuticals; including antibiotics, vaccines and brochodilators; having removable parts for adaptability to diverse pressurized inhalant pumps and is furthermore; an interchangeable platform for other pulmonary therapeutic devices.
Owner:RAPHA INST FOR HEALTH
Method and system for countering hostile activity aboard an airplane
InactiveUS6696928B1Not limitedElectric signal transmission systemsDigital data processing detailsJet aeroplaneWhole body
A method of countering terrorism or hostile activity in an airplane by using a built-in defense system within the aircraft. The defense system includes chemical sprays, laser guns, and pre-programmed sound alarm systems. The aerosol chemicals range from benign fogging agents to non-lethal incapacitating agents from the categories of inhalants, general anesthetics, and irritants. Any of the systems can be used singly or in any combination. These systems can be activated manually from the control panel in the cockpit, or via a remote wireless system by the flight crew from anywhere within the plane. Such activation being password and code protected.
Owner:BOVEJA BIRINDER R +1
Inhalant Formulation Containing Sulfoalkyl Ether Cyclodextrin and Corticosteroid Prepared from a Unit Dose Suspension
InactiveUS20110008325A1Improve solubilityImprove stabilityBiocideDispersion deliveryNebulizerCyclodextrin
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Atomized inhalant for treating respiratory system diseases
The invention discloses an atomized inhalant for treating respiratory system diseases. The atomized inhalant mainly prepared from, by mass, 1%-2% of 1,8-cineole, 1%-4% of solubilizers and 20%-90% of water. The inhalant is used through the steps that a medicine solution is atomized into small particles, and the small particles are inhaled into the nose, the throat, the respiratory tract and the lung, so that the medicine is deposited on the lesions to treat the diseases. The atomized inhalant for treating the respiratory system diseases treats patients who suffer from acute and chronic faucitis, rhinitis, asthma, obstructive (spastic) bronchitis, chronic bronchitis, bronchiectasia, mucoviscidosis, cysticfibrosis and chronic obstructive pneumonia and need to humidify the tracheae and dilute the sputum. The inhalant has the advantages that the effect is achieved quickly, the medicine using amount is little, use is convenient, and the safety is high.
Owner:北京神州泰洁生物科技有限公司
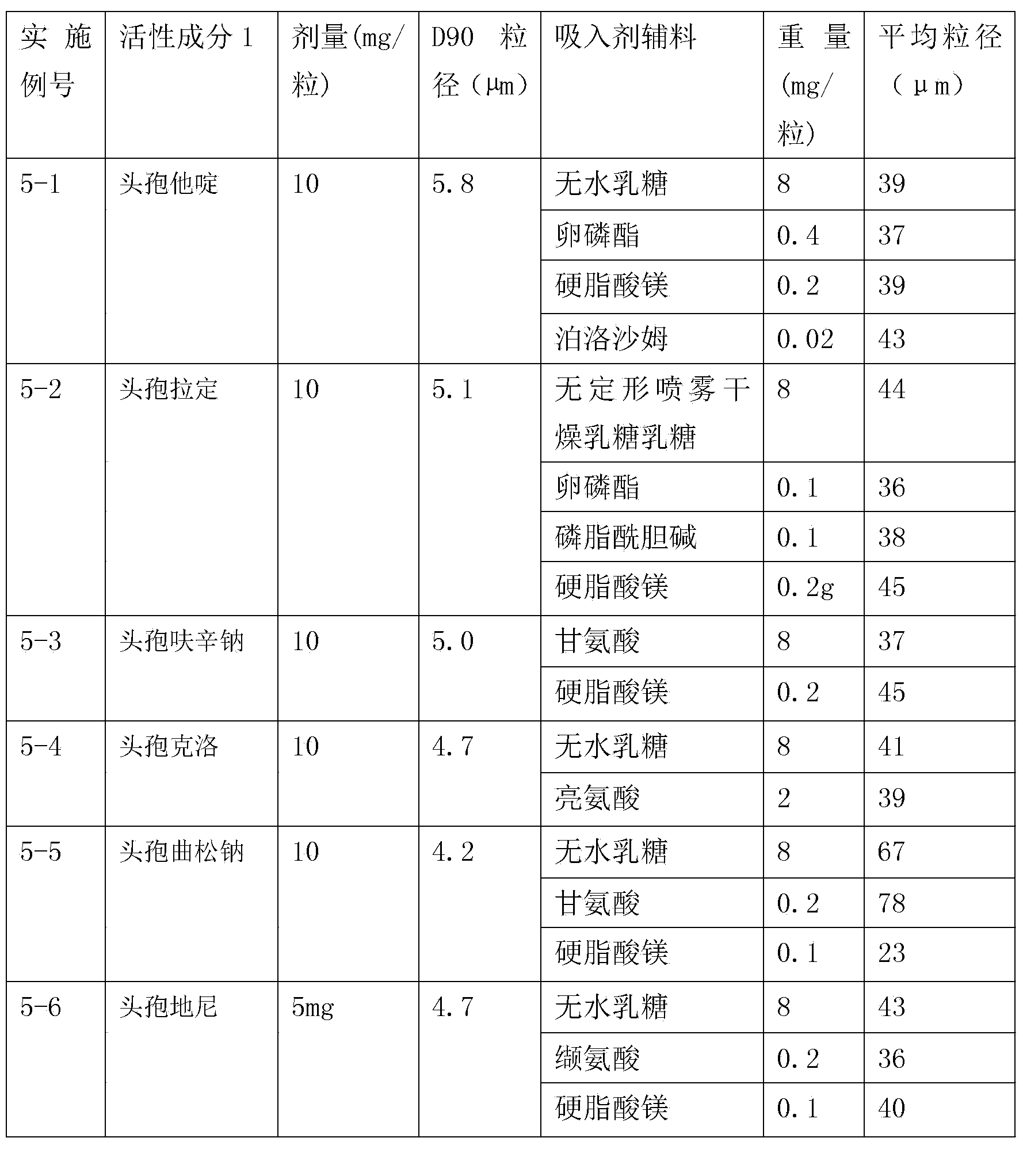
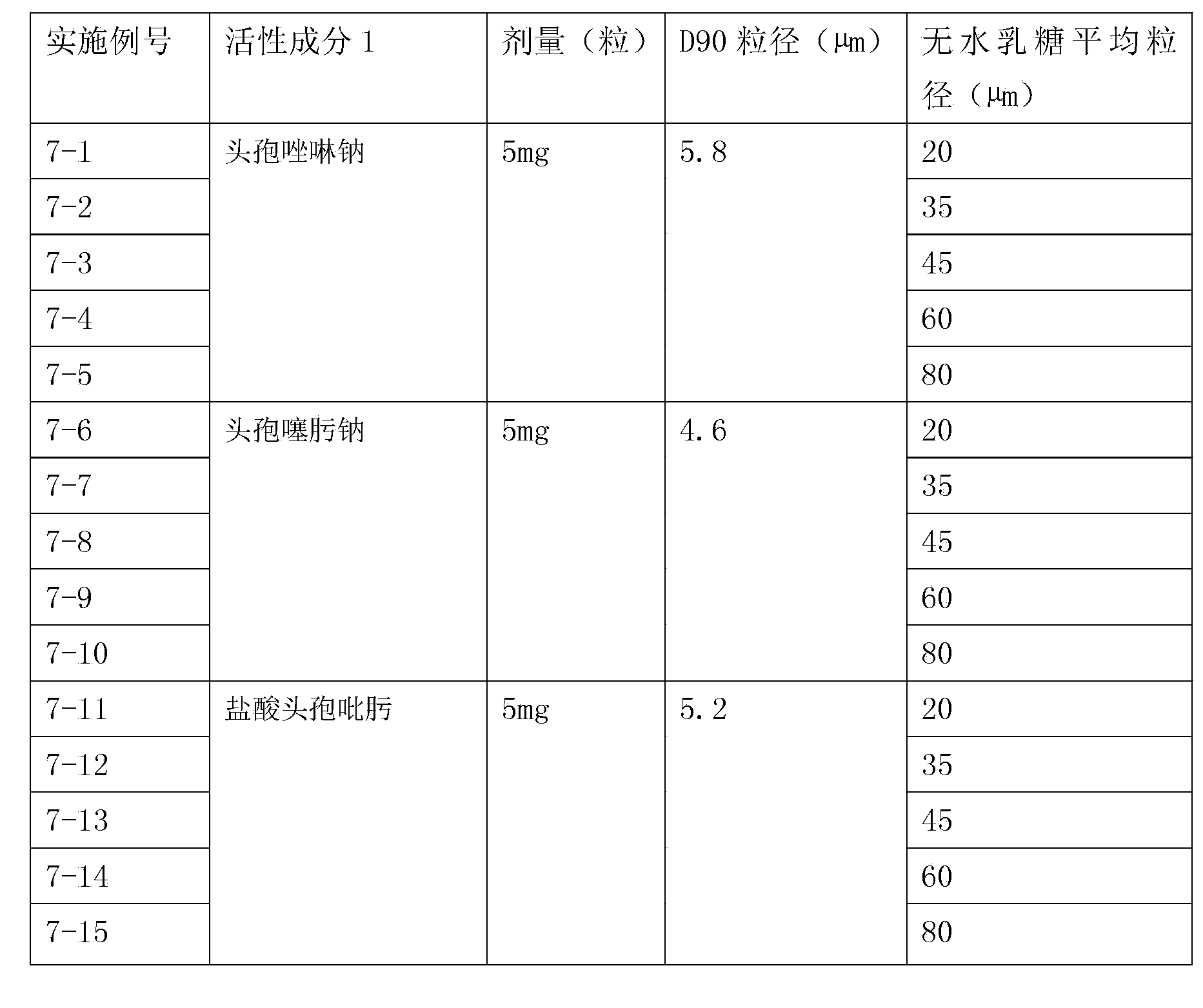
Inhalation preparation containing cephalosporin antibiotic
The invention relates to an inhalation preparation containing cephalosporin antibiotic and applications thereof. The cephalosporin antibiotic is made into an inhalation dosage form, and thus the preparation can treat pulmonary bacterial infection with less dosage and has a certain curative effect on asthma and chronic obstructive pneumonia.
Owner:TIANJIN JINYAO GRP
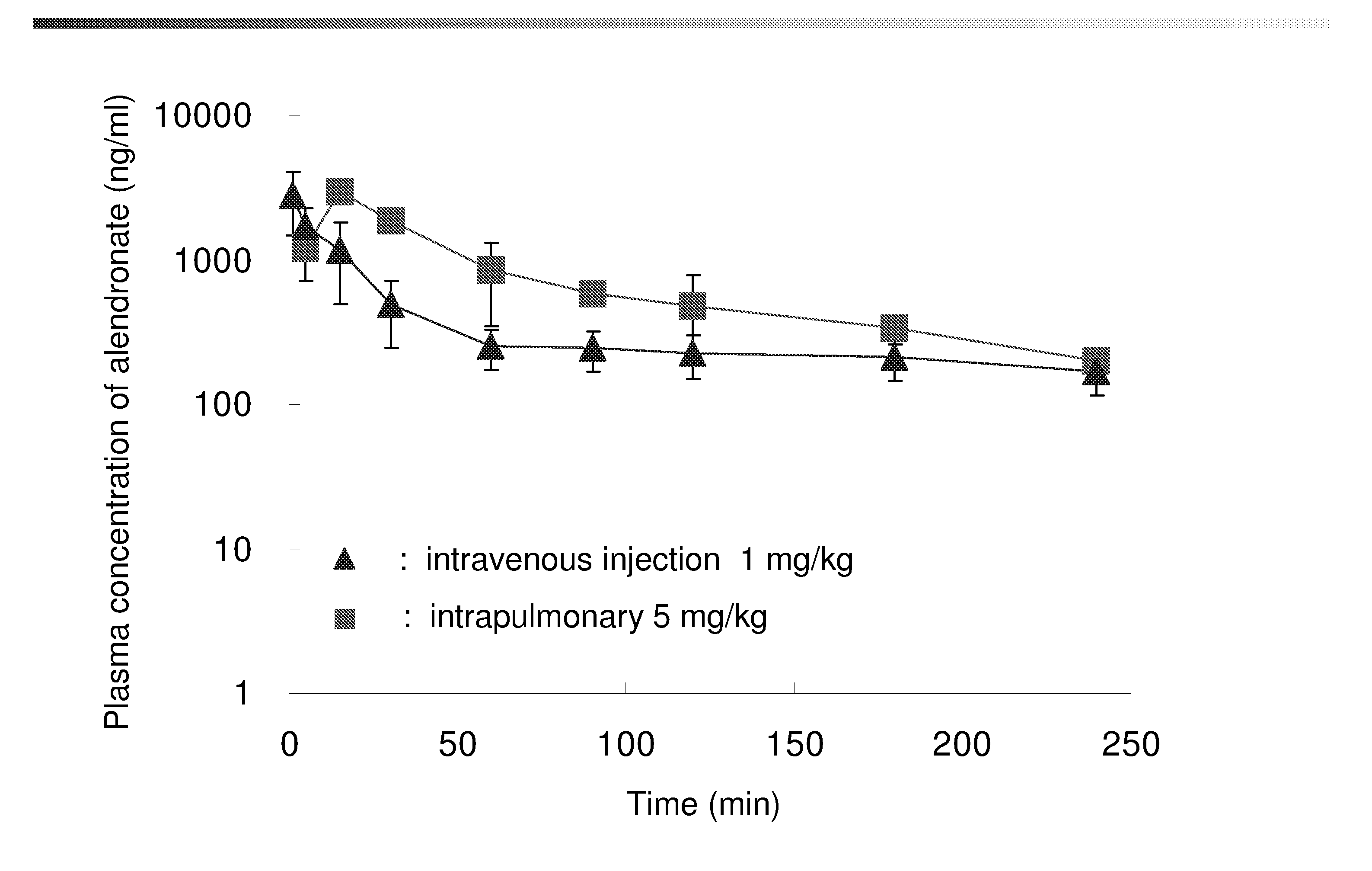
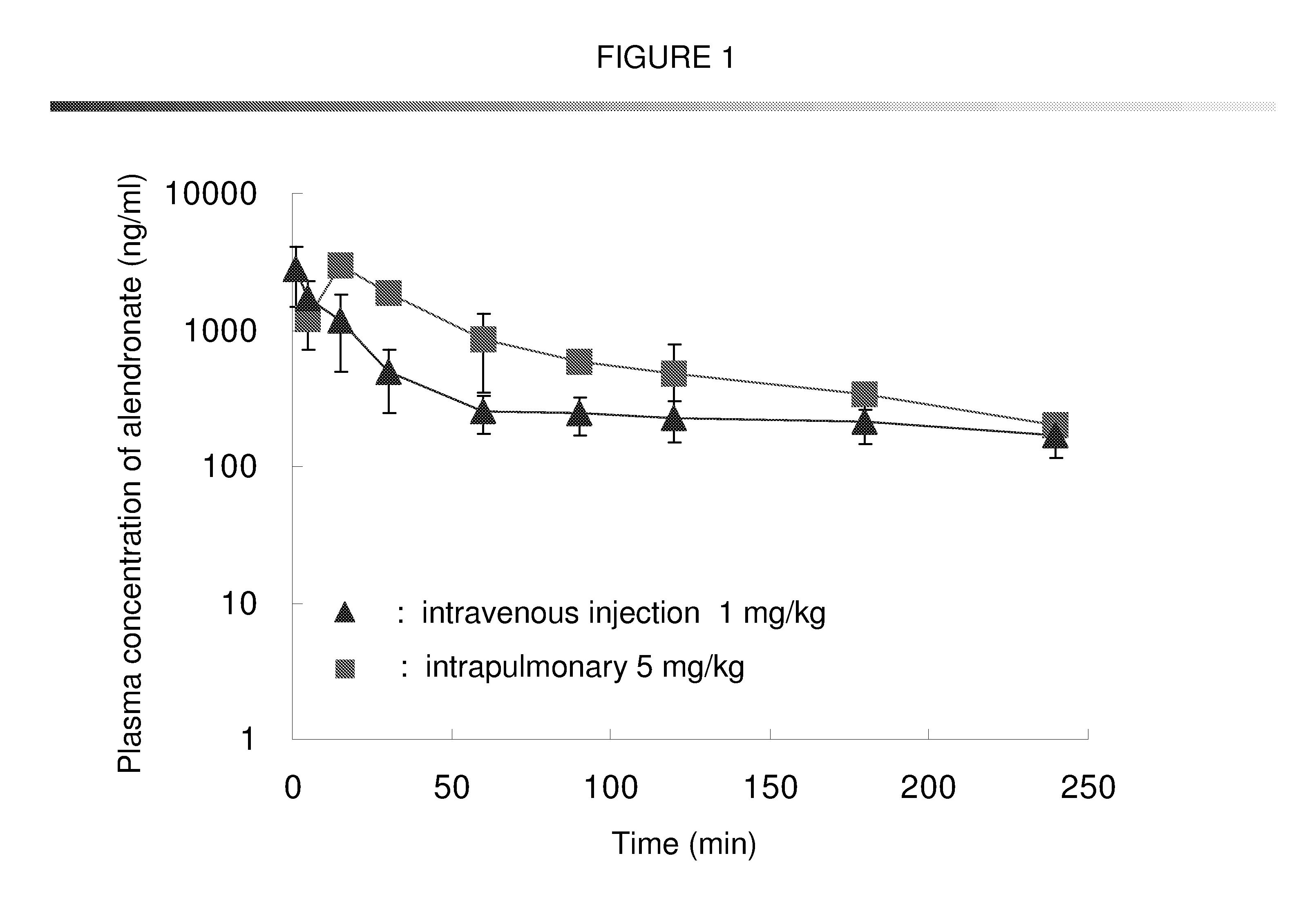
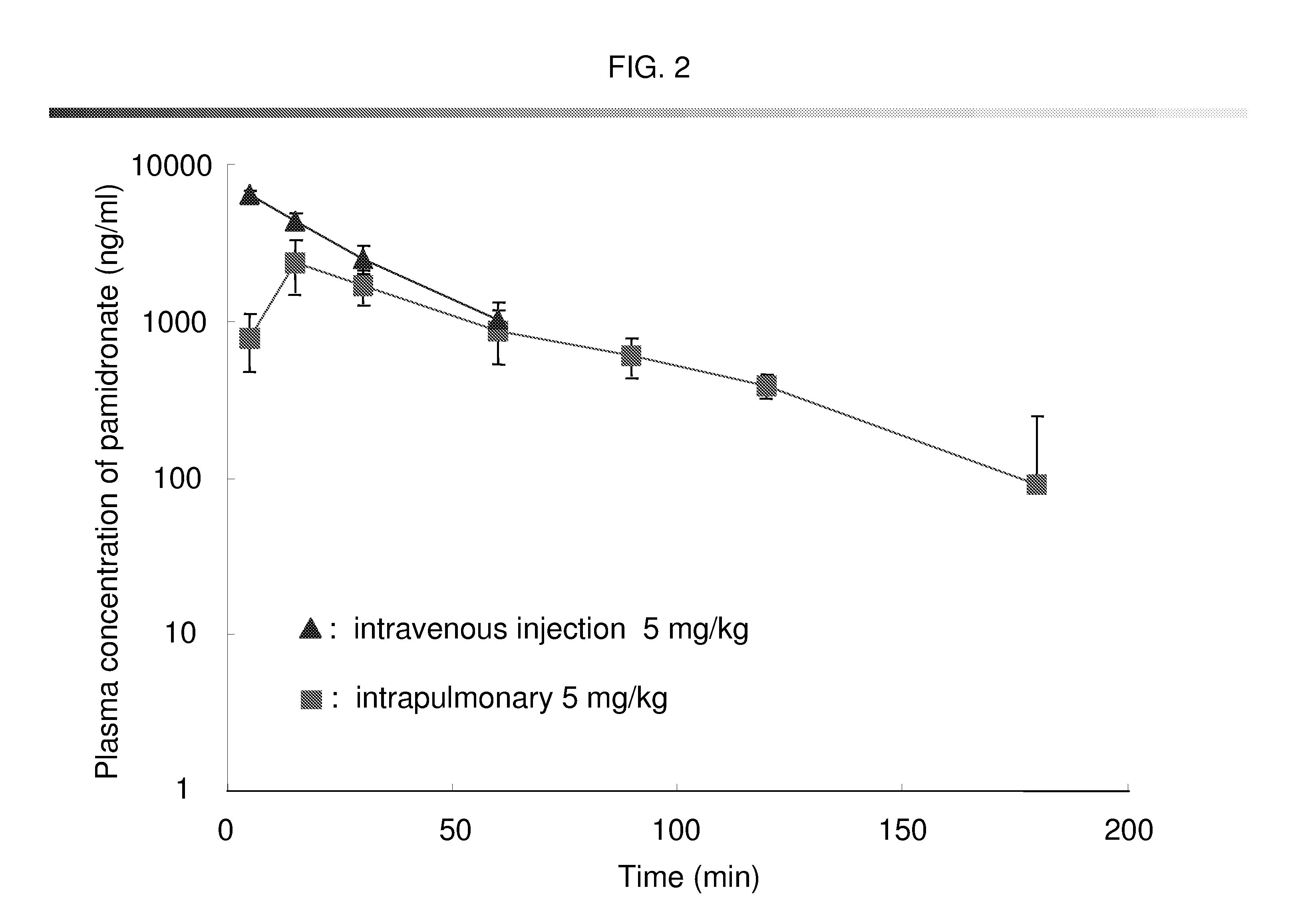
Bisphosphonate inhalant formulations and methods for using the same
The present invention provides for methods of administering by a pulmonary route an effective amount of a bisphosphonate active agent to a subject. Aspects of the invention including administering the active agent to the subject in conjunction with one or more mucosal membrane protecting agents, where the protecting agent may include one or more of a protecting enzyme and / or a protecting amino acid and / or a protecting peptide. Also provided are inhalant compositions for use in practicing methods according to embodiments of the invention. Methods and compositions according to embodiments of the invention find use in a variety of different applications, including but not limited to, the treatment of bone adsorption disease conditions.
Owner:TEIKOKU PHARMA USA INC
Inhalant dispensing system and apparatus
ActiveUS20200147325A1Monitor the user's inhalant consumptionMedical devicesMedical atomisersInhalationAerosolize
An inhalant dispensing system is provided, comprising an inhalant delivery apparatus having a main body, a lower body coupled to the main body and comprising a mouthpiece, and an inhalant delivery mechanism disposed within the main body and configured to deliver an aerosolized solution to the mouthpiece for user inhalation; and a lock out system configured to selectively prevent delivery of the aerosolized solution to the mouthpiece. The inhalant delivery apparatus may be assembled by inserting a portion of the inhalant delivery mechanism into the main body. The lower body may be rotatably coupled to the main body and moveable between a dispensing position and a storage position. The system may be used to control and monitor dosages of a solution contained within a smart canister and administered from the canister via an inhaler, to prevent accidental or unwanted usage of the canister and / or over-dosing.
Owner:LOOP LABORATORIES LLC
Alpha-interferon dry powder inhalant for pigs and method for producing same
InactiveCN101513524AExtended shelf lifeGood chemical stabilityPeptide/protein ingredientsPharmaceutical delivery mechanismEvaporationInterferon alpha
The invention discloses a formulation of an alpha-interferon dry powder inhalant and a method for producing the same. The method comprises the following steps: adding a protective agent mainly comprising tween-80 into an alpha-interferon first; preparing the mixture into the dry powder inhalant mainly comprising the alpha-interferon through rotary evaporation and vacuum freezing and drying; and filling the dry powder inhalant into a rotary inhaler to prepare a finished product. The method prolongs the storage time of the interferon, and the interferon is convenient to use and advantageous for absorbing, thus the clinical using effect of the alpha-interferon is brought into play better.
Owner:TIANJIN RINGPU BIO TECH
Isoniazid dry powder inhalant for treating pulmonary tuberculosis
ActiveCN112336703AHigh drug loadingHigh drug delivery rateAntibacterial agentsPowder deliveryIsoniazidTuberculosis bacillus
The invention relates to an isoniazid dry powder inhalant for treating pulmonary tuberculosis. The isoniazid dry powder inhalant comprises isoniazid micro-powder and comprises or does not comprise carrier micro-powder; the particle size of the isoniazid micro-powder is treated to 0.1-10 mu m by a fluidized bed supersonic jet milling method or a spray drying method; preferably, auxiliary materialsincluding leucine, mannitol or phospholipid are added into the dry powder inhalant so as to enhance the pelletizing rate of particles; and furthermore, auxiliary materials including magnesium stearate, mannitol, leucine and / or lactose are added so as to improve the flowability of the medicine and reduce the particle agglomeration phenomenon. The preparation process of the isoniazid dry powder inhalant is simple; and the isoniazid dry powder inhalant with relatively high stability can be prepared. The dry powder inhalant can effectively deliver drugs to pulmonary alveoli so as to effectively kill and inhibit tubercle bacillus in respiratory tracts, and is particularly suitable for treating pulmonary tuberculosis patients with impaired liver functions.
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
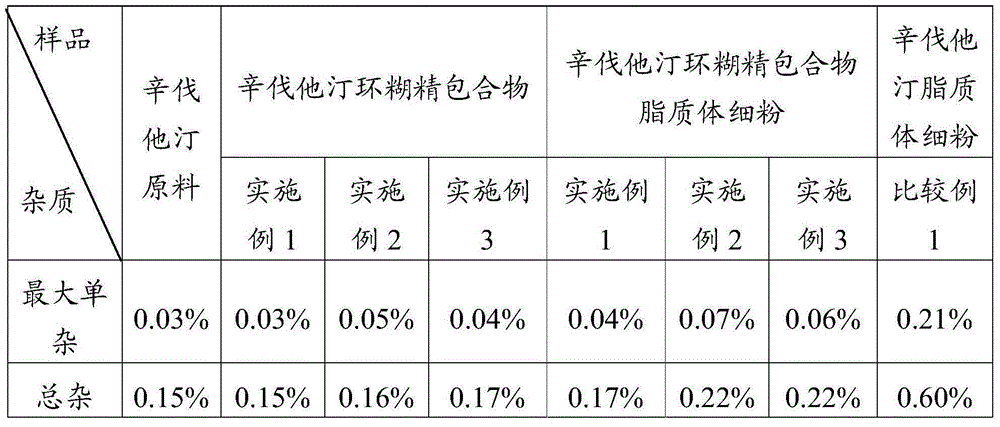
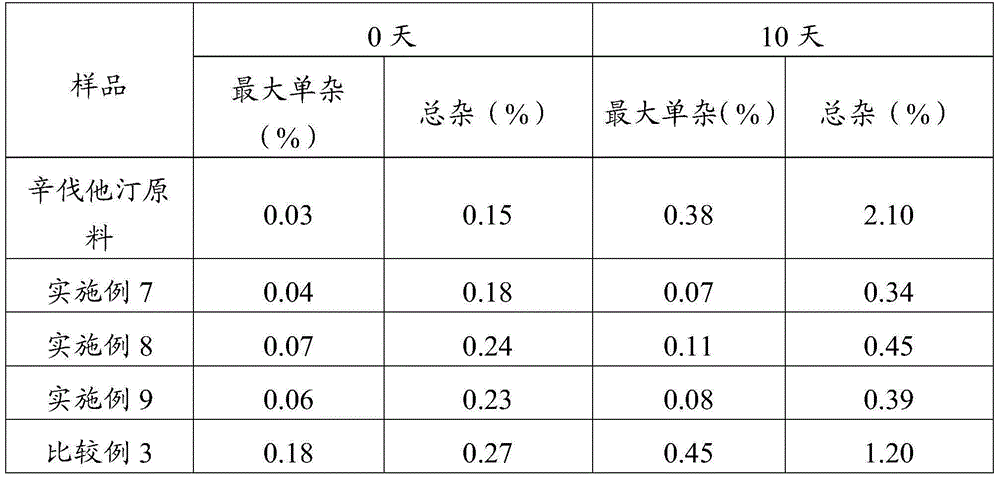
Simvastatin dry powder inhalant and preparation method thereof
ActiveCN105078931AIncrease the amount of deposition in the effective partImprove efficacyOrganic active ingredientsPharmaceutical delivery mechanismCyclodextrinCurative effect
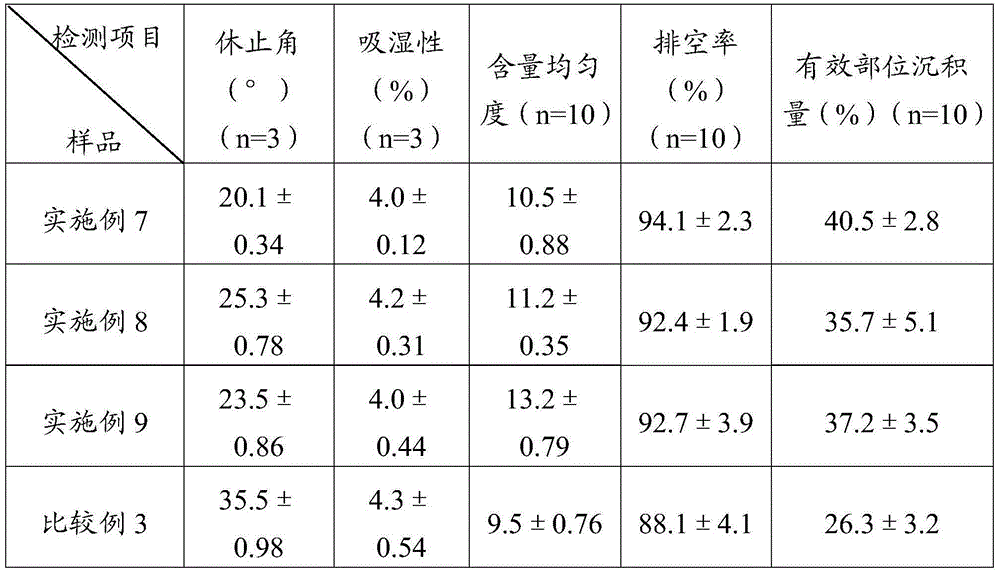
The invention provides a simvastatin dry powder inhalant. The simvastatin dry powder inhalant comprises a simvastatin liposome compound and pharmaceutically acceptable auxiliary materials. The simvastatin liposome compound is obtained by compounding cyclodextrin-coated simvastatin with phospholipid. The invention further provides a preparation method of the simvastatin dry powder inhalant. According to the simvastatin dry powder inhalant, the cyclodextrin-coated simvastatin and the phospholipid are compounded into the simvastatin liposome compound which is then compounded with the pharmaceutically acceptable auxiliary materials to obtain the dry powder inhalant; after pulmonary administration of the simvastatin dry powder inhalant, effective part deposition amount of simvastatin can be increased remarkably, so that the efficacy of pulmonary simvastatin administration on treatment of pulmonary diseases such as asthma and chronic pulmonary obstruction is improved. Experimental results show that, by the simvastatin dry powder inhalant, the effective part deposition amount is increased remarkably and curative effect on the pulmonary diseases is improved.
Owner:GUANGZHOU GONGHE MEDICINE TECH
Non-aqueous composition having drug carried therein, and method for producing same
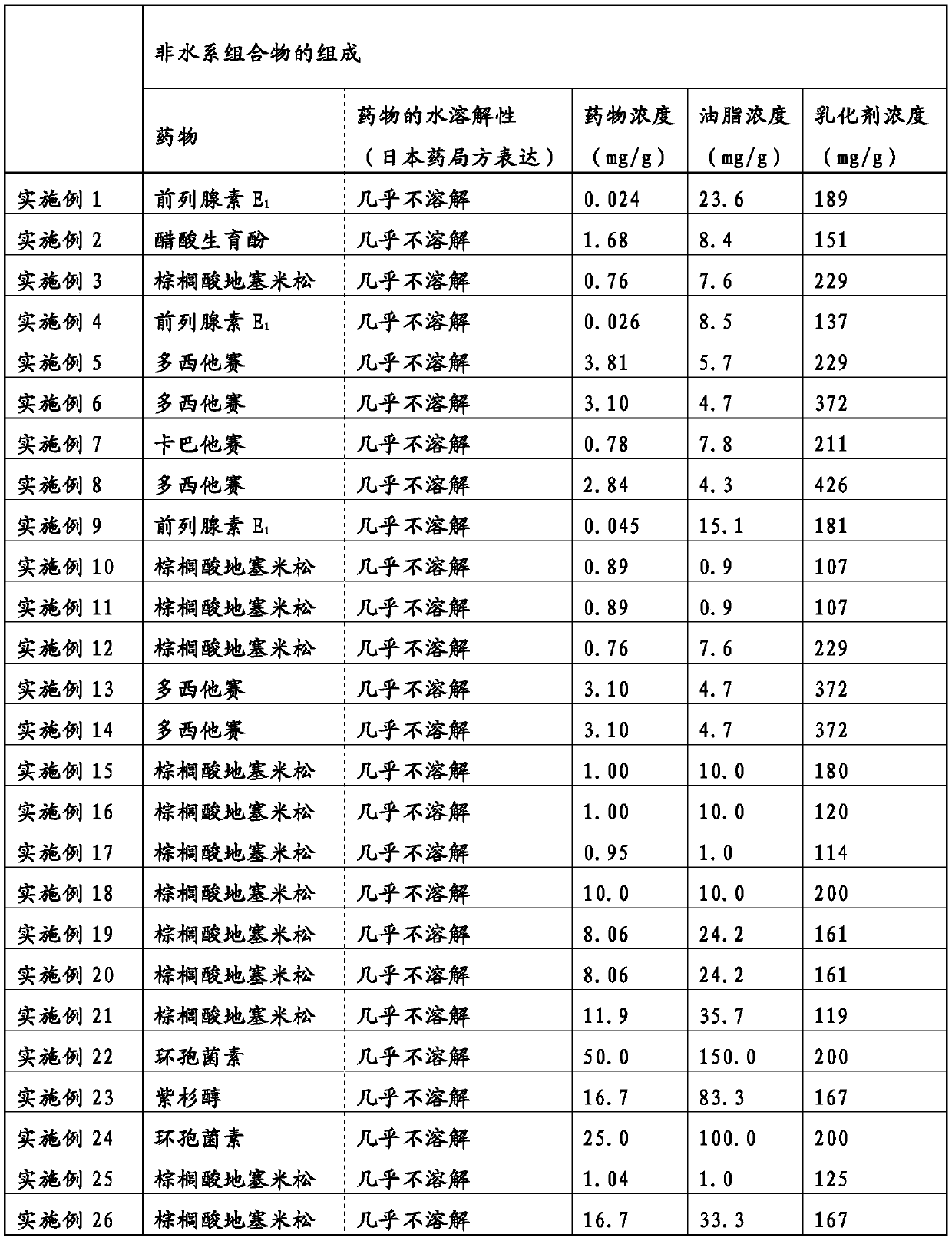
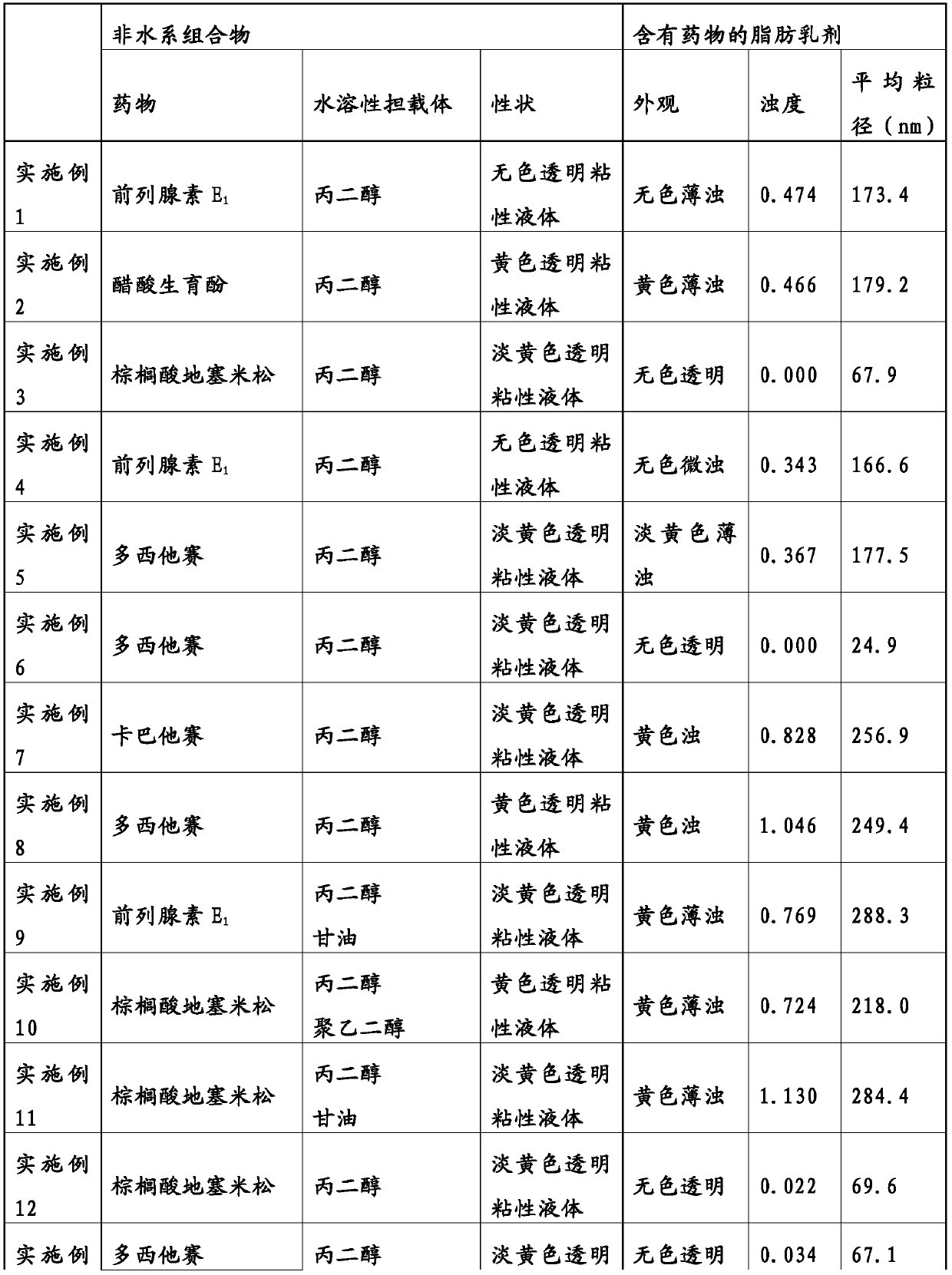
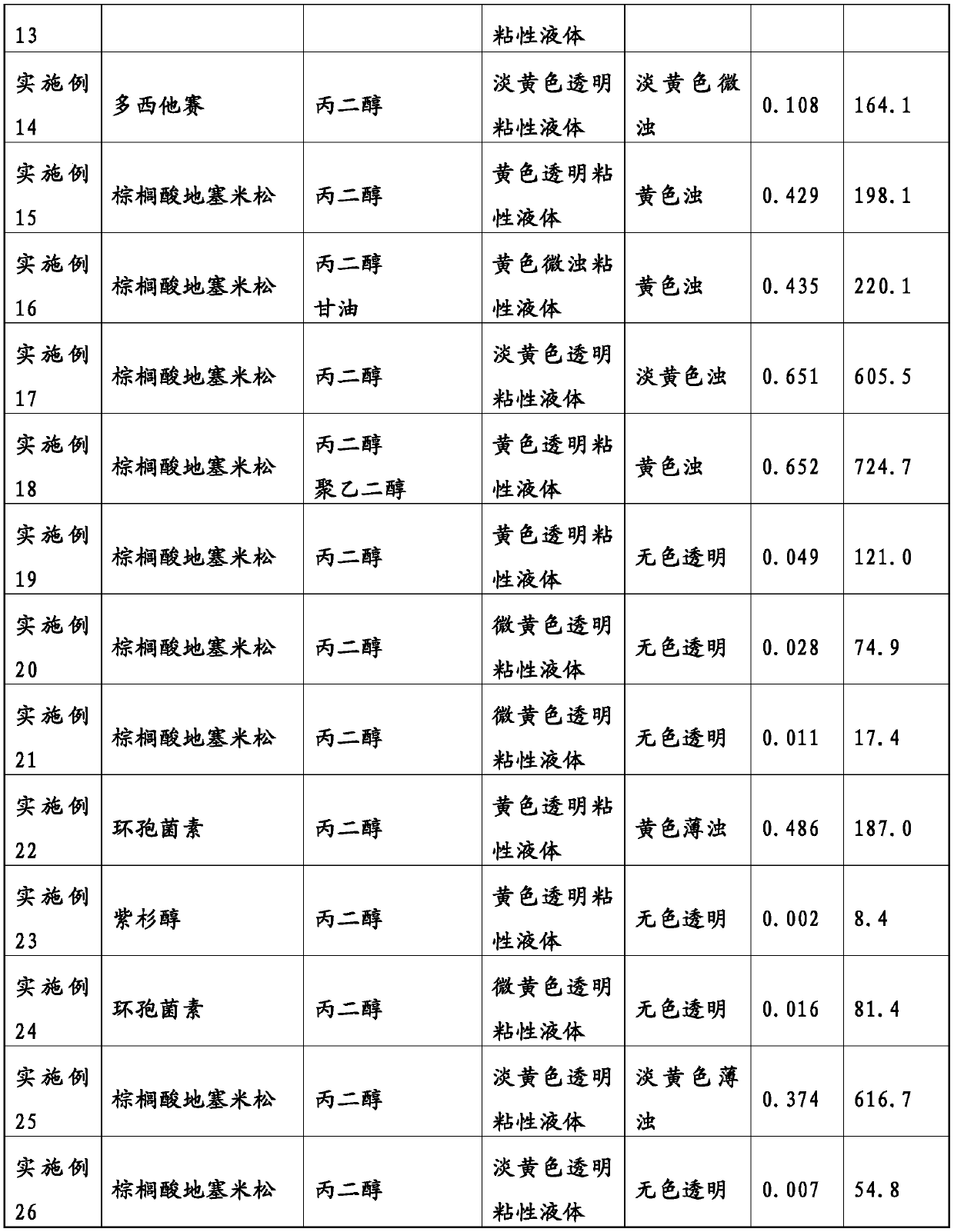
The present invention addresses the problem of providing: a non-aqueous composition having a drug carried therein, which can be prepared by mixing a drug-containing fat emulsion that can be used as aninjectable solution, an eye drop, a nasal spray, an inhalant and the like with an aqueous medium upon use without needing to produce a drug-containing fat emulsion in advance; and a method for producing the non-aqueous composition. The non-aqueous composition having a drug carried therein according to the present invention, which is a solution for the problem, is characterized in that componentsare dissolved in a polyhydric alcohol that serves as a water-soluble carrier in such a manner that the content of an oil or fat can be 0.05 to 250 mg / g, the ratio of the content of a poorly-water-soluble drug to the content of the oil or fat (i.e., (poorly-water-soluble drug) / (oil or fat)) can be 0.0001 to 50 by weight (provided that the total content of the poorly-water-soluble drug and the oil or fat is up to 300 mg / g) and the content of an emulsifying agent can be 20 to 500 mg / g.
Owner:技术防卫株式会社
Etimicin sulfate liposome inhalant and preparation method thereof
PendingCN111789828AGood biocompatibilityExtended stayAntibacterial agentsOrganic active ingredientsOrganic solventPhospholipid
The invention discloses an etimicin sulfate liposome inhalant and a preparation method thereof. The liposome inhalant is prepared from 1 part by weight of etimicin sulfate, 15-20 parts by weight of phospholipid, 125-150 parts by weight of a buffer solution and a proper amount of auxiliary materials. The pH value of the inhalant ranges from 6.2 to 6.4. The preparation method comprises the followingsteps of mixing the etimicin sulfate, the phospholipid and an organic solvent, performing dissolving, performing concentration to remove the organic solvent, adding the buffer solution, performing shaking slightly, and carrying out high-pressure homogeneous cutting to obtain etimicin sulfate liposome; and adding a proper amount of auxiliary materials, and performing dissolving, filtering and filling. The inhalant has an excellent targeting effect; compared with a common injection, the inhalant has the advantages that the bioavailability is higher, the concentration of drugs at toxic parts canbe reduced, and the safety of the drugs is greatly improved. The preparation method is simple and feasible and is suitable for industrial production.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA
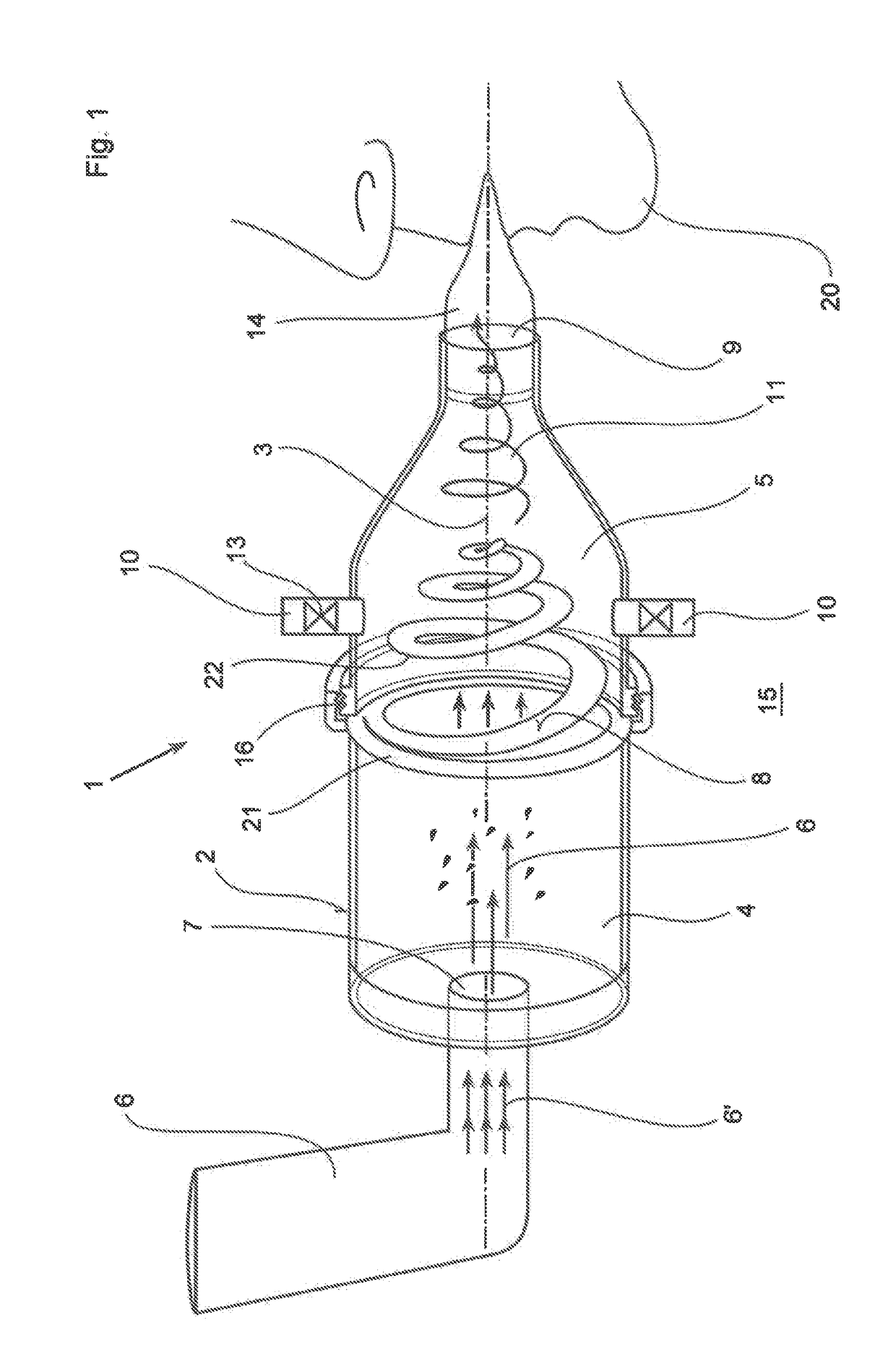
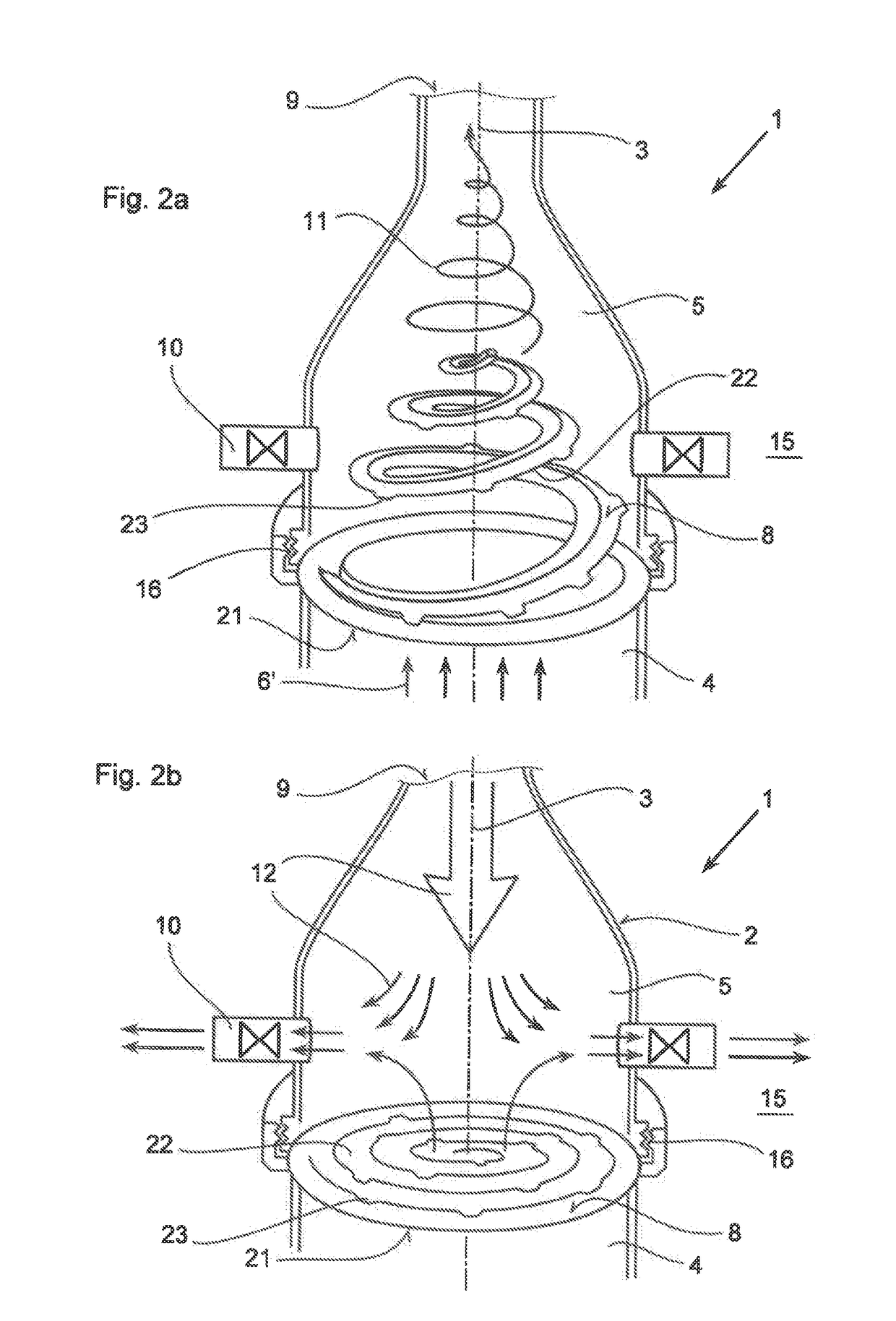
Inhalation system
In an inhalation system (1) for inhalation of a dosing aerosol (6′), comprising:a dosing device (6) which delivers the aerosol (6′)a container (2) in which one or more chambers (4, 5) separated from one another and connected together during inhalation by means of one or more valves (8) are provided,with an inlet opening (7) emerging into the first chamber (4), into which opening the dosing device (6) is inserted,with the second chamber (5) having a first passage opening (9) which interacts with the respiratory openings of a human being (20),with the second chamber (5) having a second passage opening (10) that is connected to the surrounding air (15) and through which a positive pressure in the second chamber (5) can escape into the surrounding air (15) during exhalation, a simple and hygienic application of the inhalation arrangement (1) should be guaranteed and the aerosol flow should have a linear and rotating motion induced in it during the inhalation procedure.This is achieved in thatthe valve (8) is formed from an elastically deformable material, preferably silicone, that the valve (8) has a ring-shaped circumferential collar (21) that is fixed to the container (2),at least one slot-shaped notch (22) is worked into the valve (8) which is configured as a spiral running from inside to outside,that during inhalation, the valve (8) acts as a barrier to the flow of the aerosol (6′) flowing through it, as a result of which a linear motion and a spiral-shaped flow direction (11) along and about the longitudinal axis (3) of the container (2) is imposed on the aerosol (6′), channelling it into a three-dimensional spiral
Owner:R CEGLA
Composition for inhibiting respiratory viruses and method for preventing and treating respiratory viruses
PendingCN114272232AGood killing effectNot infectedAntibacterial agentsAntimycoticsDiseaseRespiratory tract disease
The invention particularly relates to a composition for respiratory viruses and a method for preventing and treating the respiratory viruses. Respiratory virus infection is very common clinically, but at present, therapeutic drugs aiming at virus infection cannot meet therapeutic requirements. The invention provides the inhalant for inhibiting respiratory viruses, prevention and treatment are carried out in a manner of inhaling high-temperature acetic acid steam, and acetic acid in the high-temperature steam can be used for inactivating the viruses in the respiratory tract by releasing hydrogen ions, so that the effects of prevention and even treatment are realized before diseases occur. In addition, the composition can also be used for inhibiting fungi and bacteria in vitro and preventing skin diseases and respiratory diseases caused by the bacteria and the fungi. The prevention and treatment method is easy to implement and economical in cost, and has important clinical application value in popularization and application.
Owner:SHANDONG UNIV
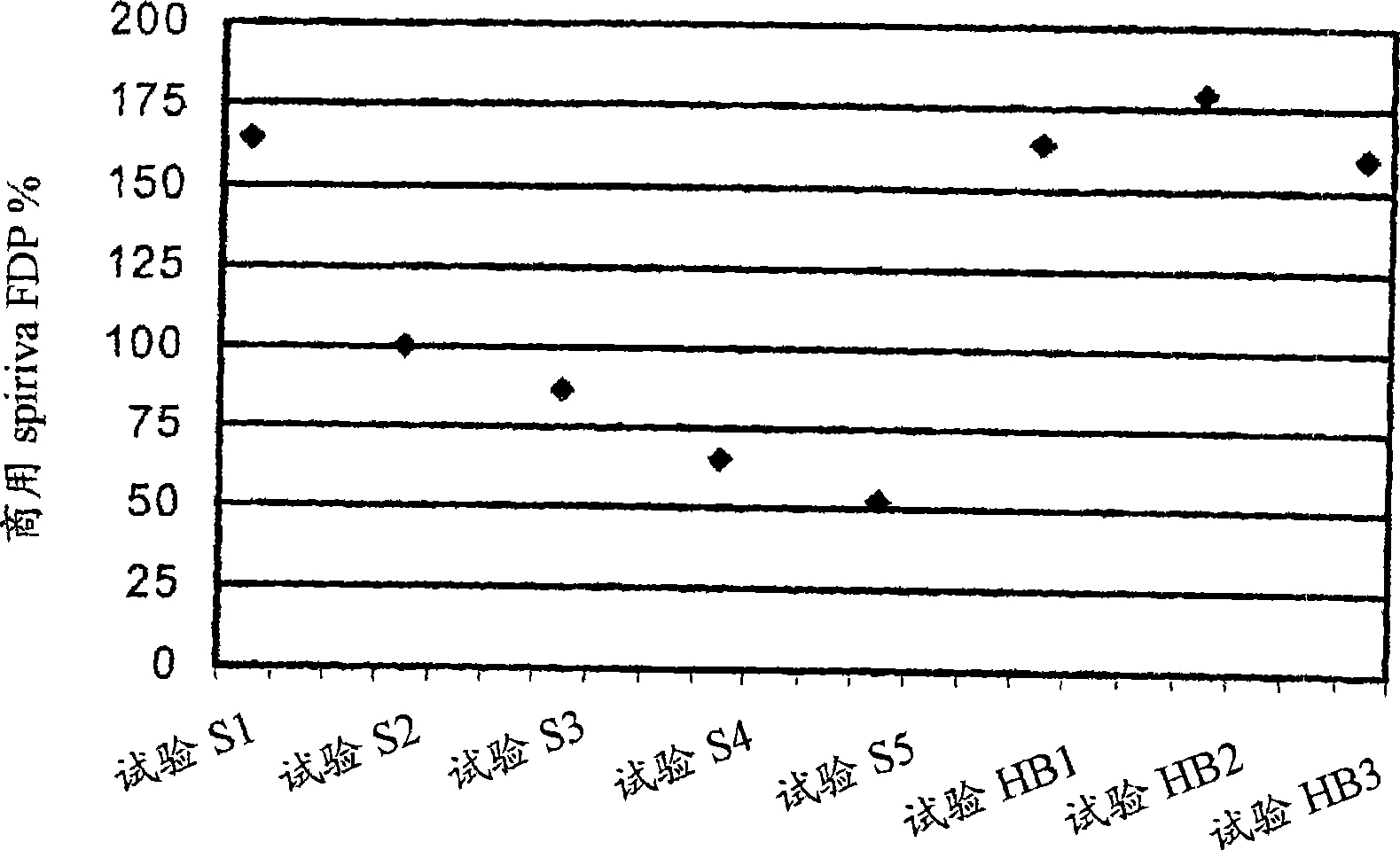
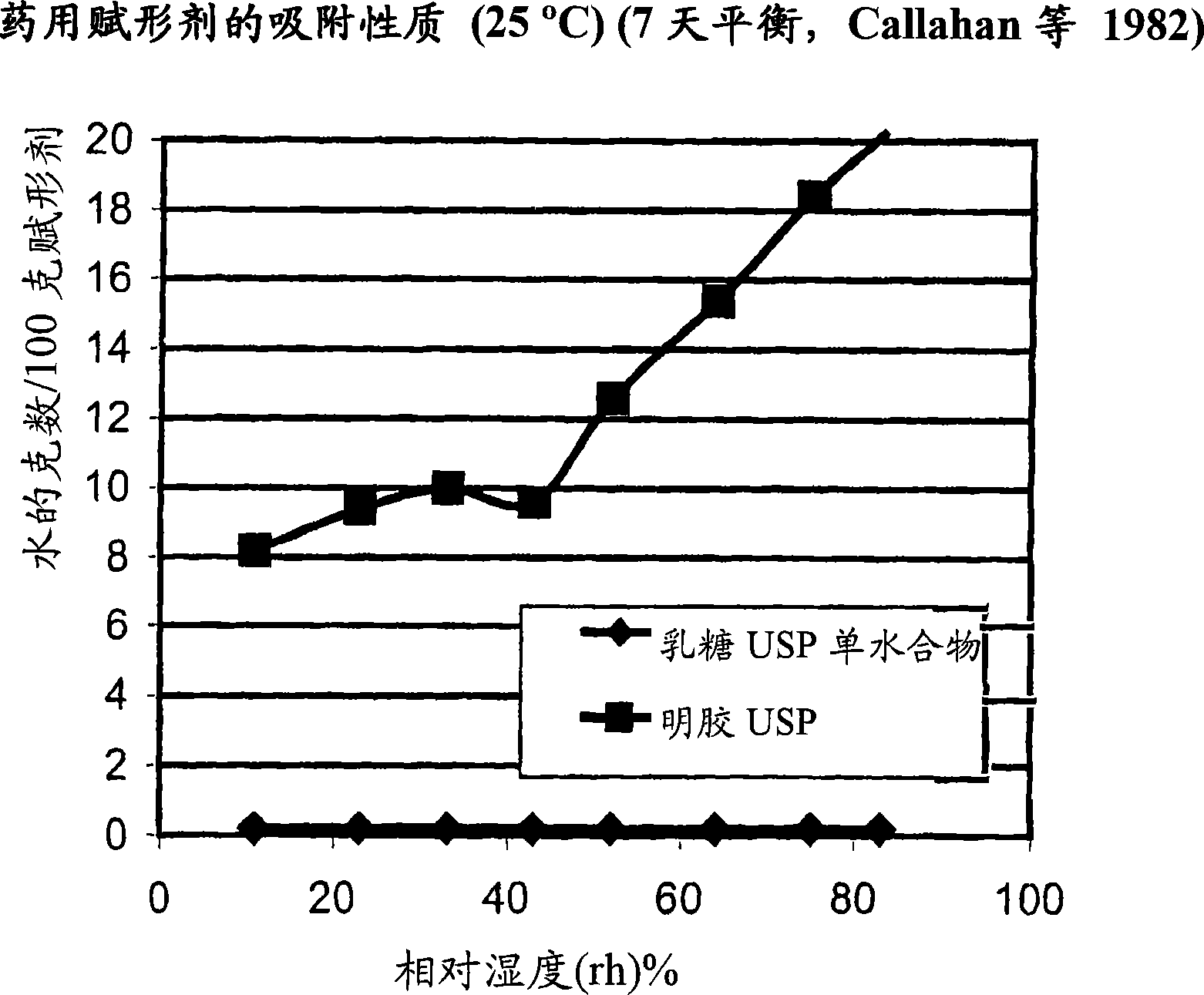
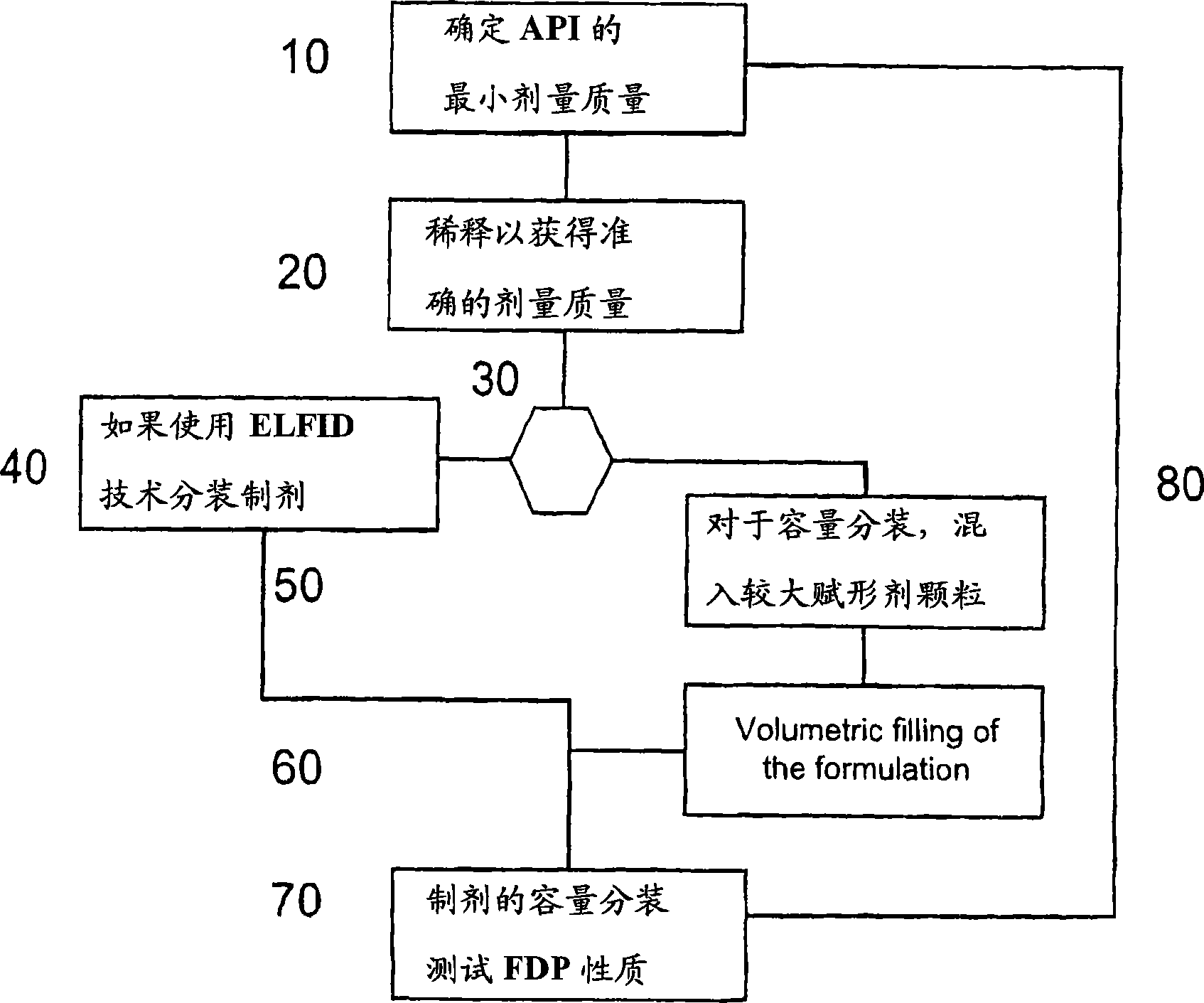
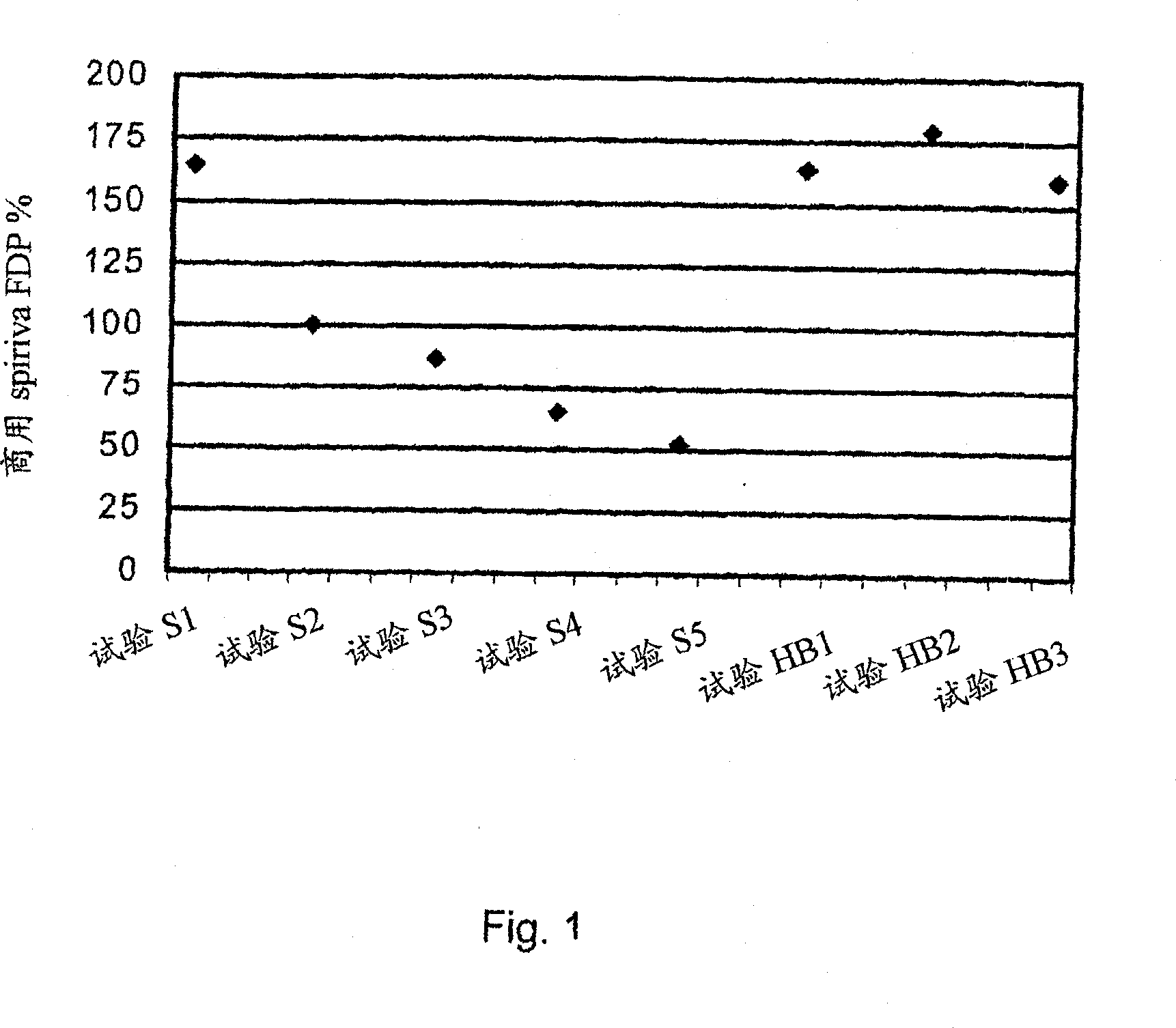
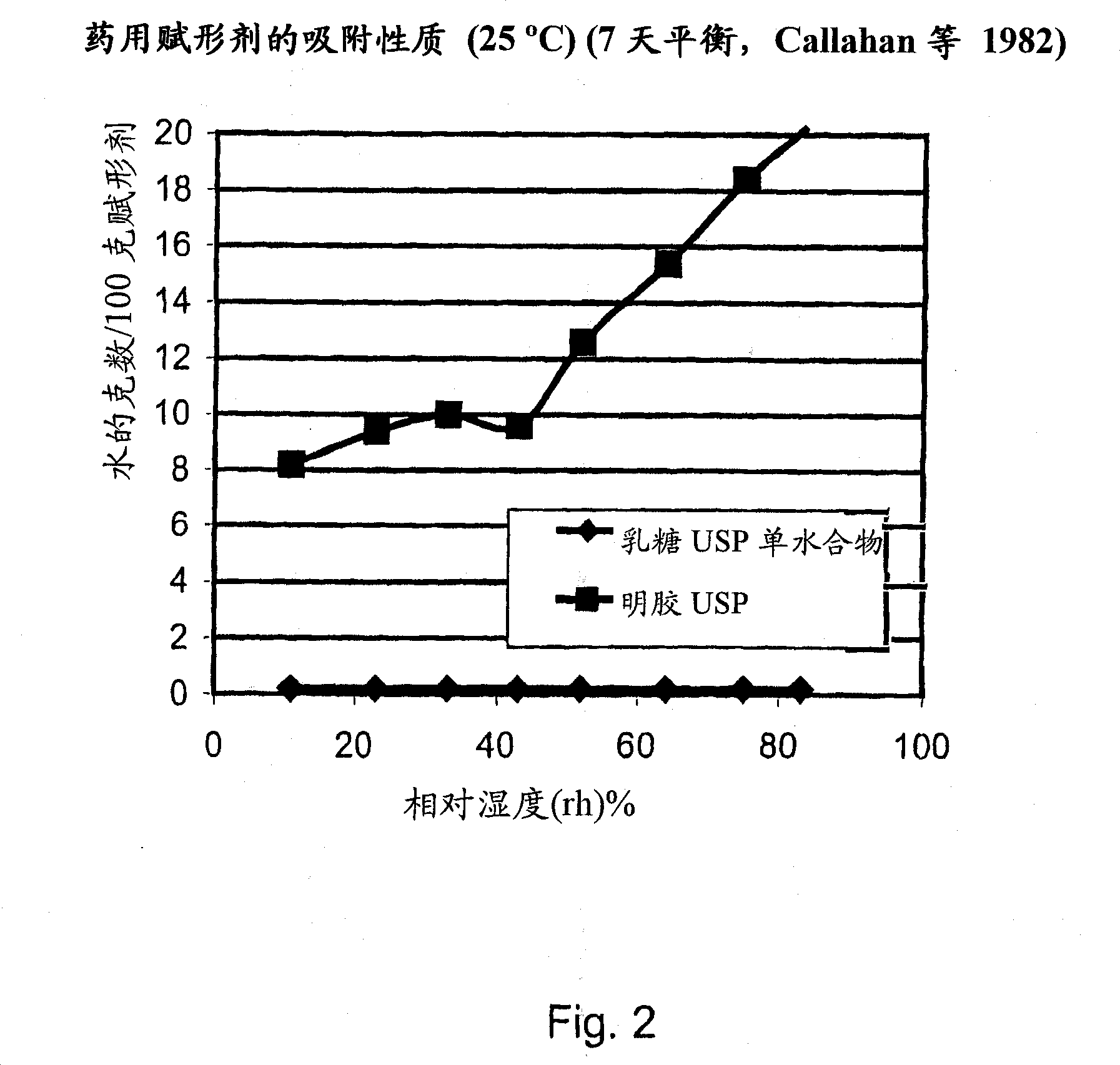
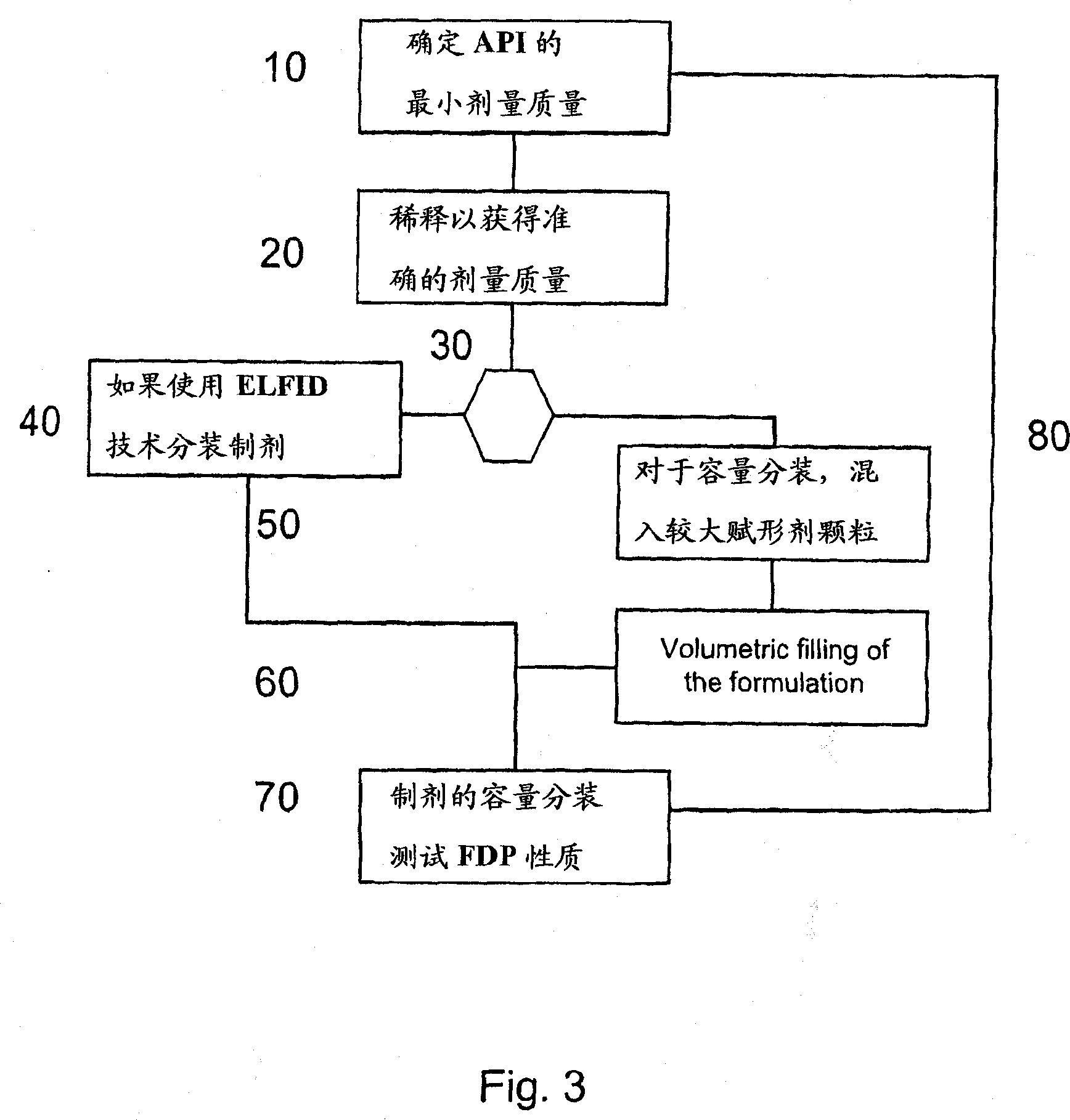
Pre-metered dry powder inhaler for moisture-sensitive medicaments
The invention discloses a medical product for use in a treatment of respiratory disorders, and comprises a metered dose of a tiotropium dry powder formulation, directly loaded and sealed into a container made to act as a dry high barrier seal to prevent the capture and ingress of moisture into the tiotropium powder. The dose of tiotropium is further adapted for inhalation and the container is so tight that the efficacy of the dose when delivered is unaffected by moisture. In a further aspect of the invention a type of inhaler is illustrated, which may accept at least one sealed, moisture-tight container of a dose of tiotropium, to deliver the dose with a consistent fine particle dose, over the expected shelf life of the product.
Owner:BOEHRINGER INGELHEIM INT GMBH
Application of cyclodextrin-metal organic framework in preparation of inhalant and inhalant
PendingCN112870371AImprove the effective deposition rateImprove delivery efficiencyPowder deliveryRespiratory disorderCyclodextrinMetal-organic framework
The invention relates to application of a cyclodextrin-metal organic framework in preparation of an inhalant and the inhalant. According to the invention, cyclodextrin-metal organic frameworks (CD-MOFs) are found to be used as a carrier for preparing the inhalant for the first time, and the obtained inhalant has high lung effective deposition rate, good aerodynamic behavior, controllable particle size structure, uniform and ordered pore channels and high porosity, so that good lung drug delivery efficiency can be realized.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Oxygen therapy mask suitable for lung disease treatment
PendingCN112827042AEnsure the supply of medicineDrug deliveryRespiratory masksOxygen deliveryInhalant Solution
The invention relates to the technical field of oxygen delivery masks, and discloses an oxygen delivery mask suitable for lung disease treatment, which comprises a mask body, wherein a pipeline is mounted on one side of the mask body, a communicating pipe is mounted at the bottom end of the pipeline in a threaded manner, a three-way connector is mounted at the bottom end of the communicating pipe, and an oxygen delivery pipe is mounted on one side of the three-way connector. A three-way connector is arranged in the pipeline, a medicament pipe is arranged at the bottom end of the three-way connector, a plug is arranged in the bottom of the pipeline, a spiral groove is formed in the side wall of the plug, and a spiral channel is formed between the plug and the pipeline. According to the oxygen therapy mask, oxygen supply can be achieved while medicine gas supply is guaranteed, so that firstly, oxygen supply and medicine taking can be conducted at the same time, and secondly, due to the fact that oxygen supply can be conducted during medicine supply, the risk caused by oxygen interruption when a critical patient inhales medicine is reduced, and further due to continuous oxygen supply, the patient can be ensured to be in a relatively stable state, so that a good inhalant treatment effect is also ensured.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Compound dry powder inhalant and application thereof
ActiveCN113662952AExtended half-lifeExtension of timePowder deliveryOrganic active ingredientsPowder InhalerFibrosis
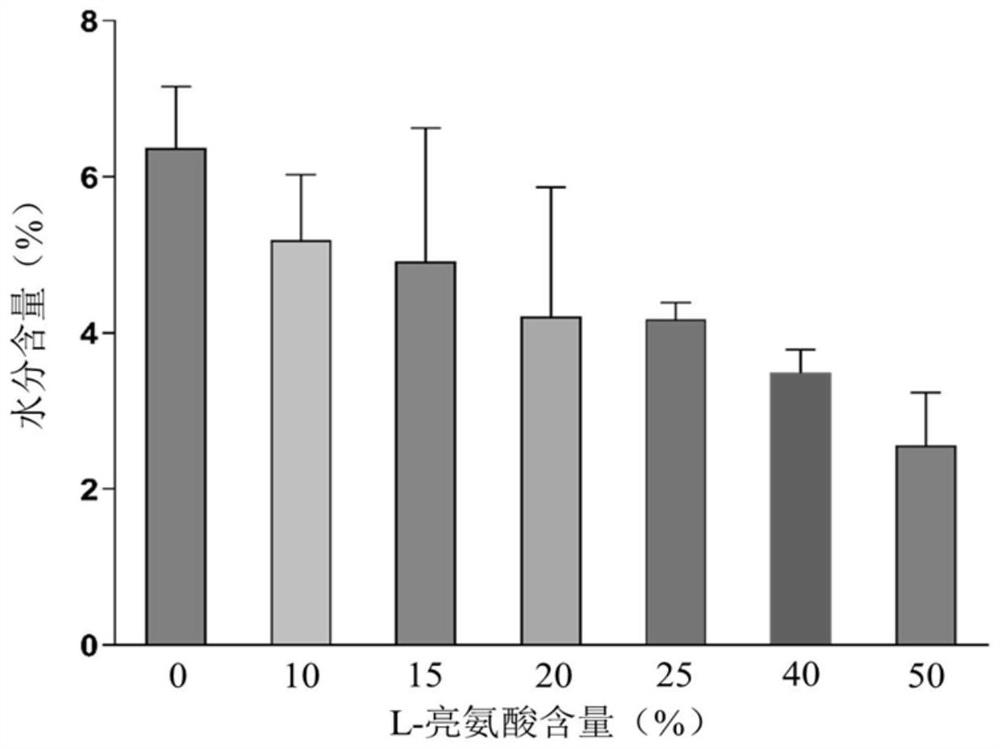
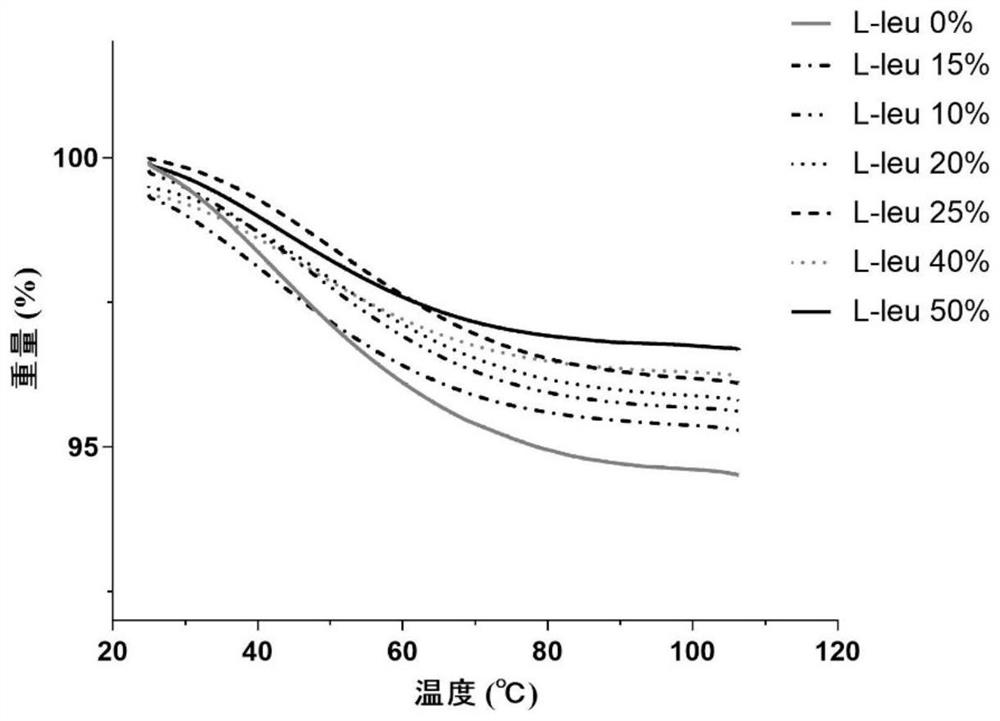
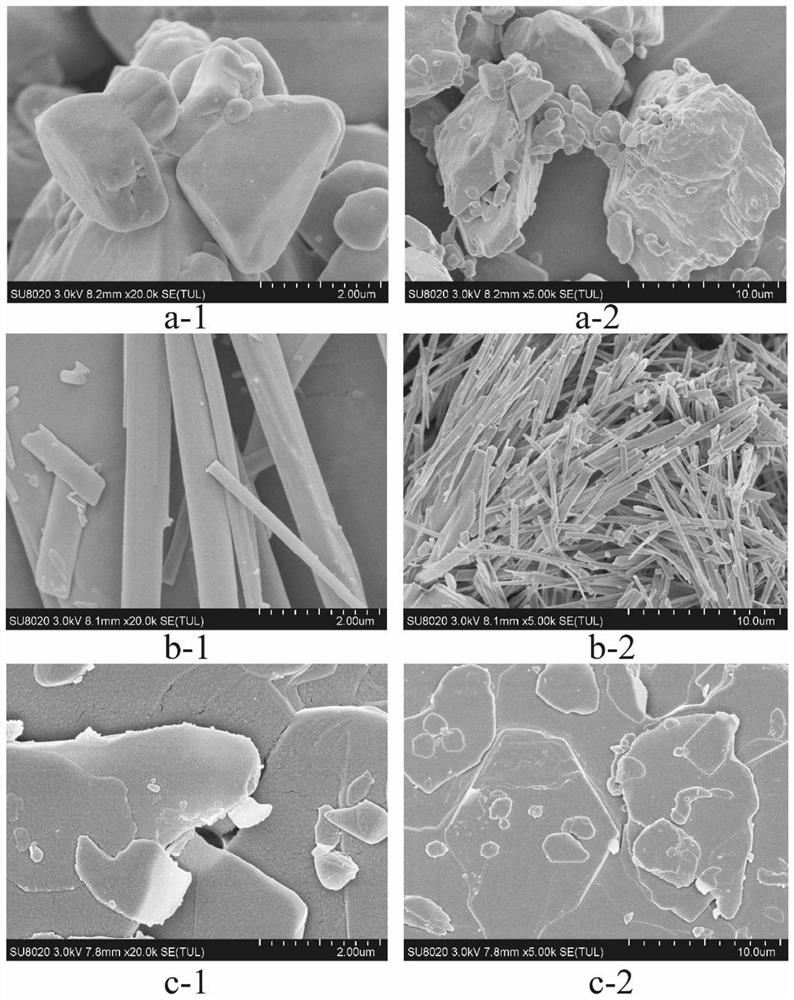
The invention provides a compound dry powder inhalant which comprises baicalin, ambroxol hydrochloride, L-leucine and phosphate, the mass of the compound dry powder inhalant is as follows: the L-leucine accounts for 0-50%, the mass of the phosphate accounts for 15-35%, the total mass of the baicalin and the ambroxol hydrochloride accounts for 15-85%, and the mass ratio of the baicalin to the ambroxol hydrochloride is 1:(0.2-2); and the Dv90 of the compound dry powder inhaler is less than or equal to 5 microns. The combination of baicalin and ambroxol hydrochloride can effectively reduce inflammation and oxidative damage of lung tissues, relieve pulmonary edema and histopathologic change, and relieve pulmonary dysfunction and fibrosis. The compound dry powder inhalant is administrated through the lung, so that the half-life period and the in-vivo residence time of the medicine in plasma are obviously prolonged, the bioavailability of the medicine in lung tissue is improved, the clearance rate of the medicine in the lung tissue is reduced, the residence time of the medicine in the lung is prolonged, and the medicine can fully play a role in the lung tissue.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Simulation device for characterizing aerodynamics of dry power inhalants in respiratory system
ActiveUS20200394937A1Reduce riskImprove accuracyComponent separationParticle size analysisMedicineProcess engineering
The present invention discloses a simulation device for characterizing aerodynamics of dry powder inhalation in respiratory system comprising: a constant temperature-and-humidity chamber, a steam and vacuum generating device and a respiratory system model arranged in the constant temperature and humidity chamber, and the constant temperature and humidity chamber and the respiratory system model are both connected with the steam and vacuum generating device; a temperature and humidity sensor is arranged in the constant temperature-and-humidity chamber and electrically connected with the steam and vacuum generating device; the respiratory system model comprises an oral cavity receiver and sample collectors, wherein inner walls of the respiratory system model are coated with a coating, the sample collectors includes a first sample collector and a second sample collector, each of the collectors is provided with 8 collecting trays.
Owner:ZHUHAI RESPROLY PHARM TECH CO LTD
Inhalant device favorable for inhalant to enter deep part of lung
ActiveCN111956918AEliminate residueReduce the risk of adverse reactionsMedical atomisersInhalatorsUse medicationInhaled drug
The invention relates to the field of inhalant devices, in particular to an inhalant device favorable for inhalant to enter deep part of lung. The device comprises a shell, the shell is provided witha storage part used for containing a medicine storage tank and an inhalation part used for inhaling medicine by a patient. The inhalant device further comprises a threshold switch, the threshold switch has a braking state and an opening state, the threshold value of the threshold switch is matched with the value of negative pressure generated in the inhalation part when the patient inhales from the inhalation part, and when the threshold switch is in the braking state, the medicine is stopped, so that the patient cannot inhale the medicine through the inhalation part; and when the value of thenegative pressure generated when the patient inhales from the inhalation part is equal to or larger than the threshold value, the threshold value switch is changed from the braking state to the opening state and kept in the opening state, and the patient can inhale the medicine from the inhalation part. In the scheme of the invention, the medicine can enter the lung at a relatively high flow rate, so that a good medicine supply effect and a good treatment effect can be achieved, and the adverse reaction risk of medicament use is greatly reduced.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
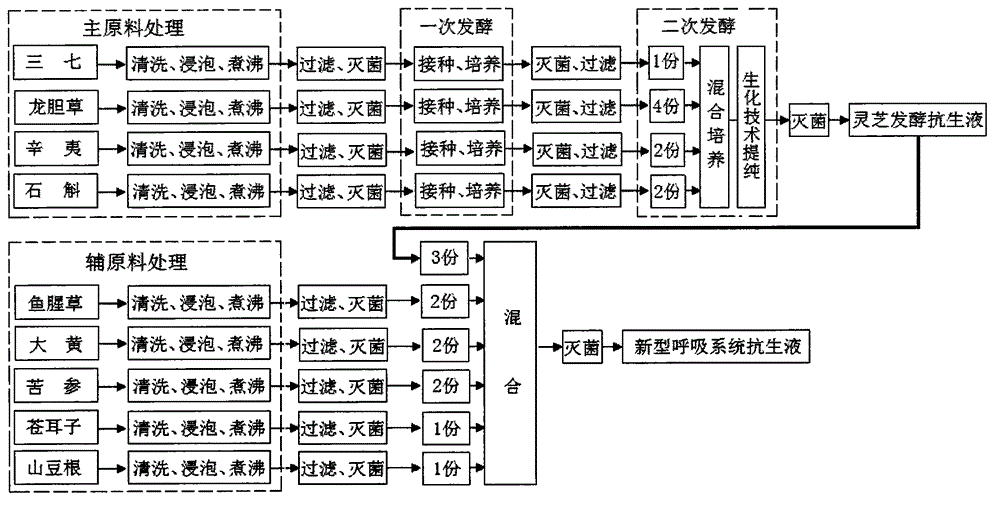
Novel respiratory system antibiotic liquid and preparation method of same
InactiveCN104825830AQuick killSolve the drawbacks in the treatment of respiratory diseasesAntipyreticAnalgesicsBiotechnologyBacterial virus
A novel respiratory system antibiotic liquid and a preparation method of the same. The multifunctional respiratory system antibiotic liquid is prepared by extracting effective components from following traditional Chinese medicine raw materials including: pseudo-ginseng, gentiana scabra, flos magnolia, dendrobe, herba houttuyniae, rhubarb, sophora flavescens, fructus xanthii and radix sophorae tonkinensis and the like, by means of a bio-fermentation engineering technology and a bio-chemical engineering technology according to a special process and a special formula. The antibiotic liquid can safely, high-effectively, quickly and synchronously kill bacteria, viruses, mycoplasma, chlamydia and the like pathogenic microorganisms causing respiratory diseases. The anti-bacterial range of the antibiotic liquid greatly exceeds a limit that conventional antibiotics only can kill and inhibit bacteria, thereby solving the defects of a chemical medicine and a traditional Chinese medicine in treatment of the respiratory diseases, so that the antibiotic liquid can be used for replacing conventional medicines. The antibiotic liquid has the functions of astringency, inflammation removal, cough calming and asthma treatment, can be prepared into an atomized inhalant and a spray of respiratory system, is convenient to use, is low in production cost, is good in effects and is free of toxic and side effects. In addition, the antibiotic liquid is free of generation of polluting substances not only in production but also in use and is very wide in market prospect.
Owner:YUNNAN MINGSHIDA SCI TECH CO LTD
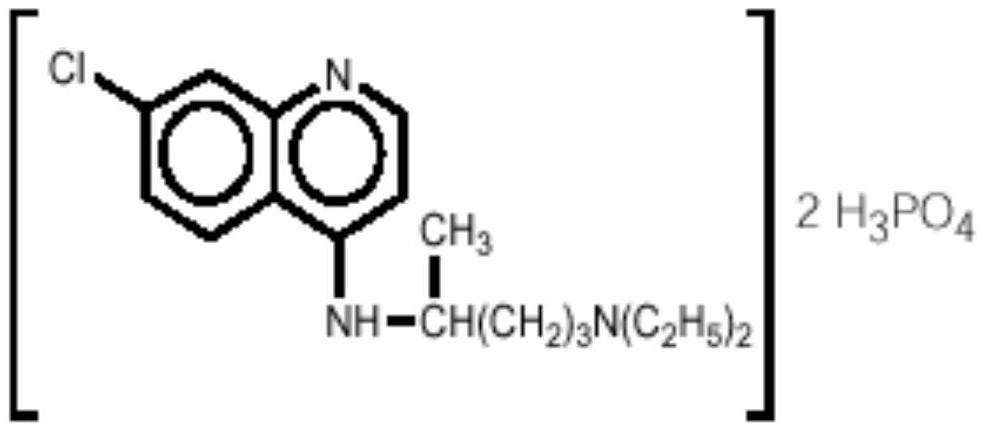
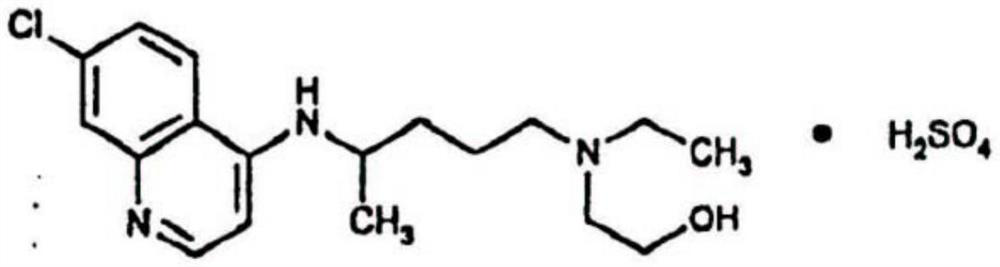
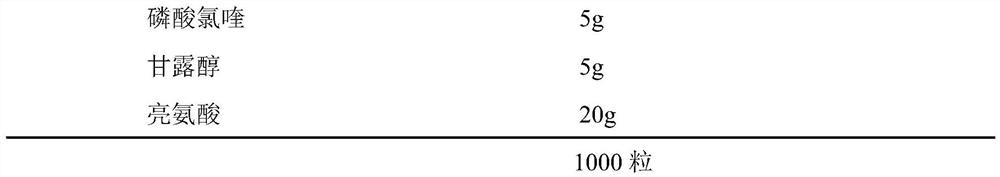
Inhalant containing chloroquine therapeutic agent and preparation method of inhalant
InactiveCN113559086AImprove stabilityImprove the protective effectOrganic active ingredientsPowder deliveryOral medicationInhalation
The invention provides a chloroquine inhalant and a preparation method thereof, and belongs to the field of medicine preparations. The inhalant comprises chloroquine phosphate or hydroxychloroquine sulfate, a stabilizer and a diluent, can improve use efficiency, has a preventive effect, such as a new epidemic situation-COVID-19, and can take effect quickly. The powder inhalation has better stability, has the effects of masking taste, enhancing stability and protecting organism biological membranes on chloroquine phosphate or hydroxychloroquine sulfate after being wrapped by the stabilizer, is absorbed through the lungs, has a high targeting effect, also can effectively avoid the first-pass effect caused by the liver during oral administration, and improves bioavailability.
Owner:DISCOVERY SHENZHEN NEW MEDICINES DEV CO LTD
Inhalant device
The invention relates to the field of inhalant devices, and specifically relates to an inhalant device. The inhalant device comprises a shell, wherein a storage part for placing a drug storing pot andan inhalation part for a patient to inhale a medicament are arranged on the shell, and a medicament cavity communicating with the inhalation part is further formed in the shell and cooperates with the drug storing pot, and the medicament enters the medicament cavity when being sprayed from the drug storing pot; and a detection device for detecting the dose of the medicament in the medicament cavity is arranged on the inhalation part. The inhalant device provided by the invention can be used for monitoring the actual dose of the medicament inhaled by a patient, and when a detection result displays that the medicament is remained in the medicament cavity, the residual medicament can be inhaled by the patient again until being completely inhaled, so that the sufficient dose of the medicamentis guaranteed, and a good treatment effect is guaranteed.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Aerosol inhalant and preparation method thereof
InactiveCN111840256ASmall particle sizeImprove bioavailabilityAntibacterial agentsOrganic active ingredientsPhenylacetic acidPharmaceutical drug
The invention discloses an aerosol inhalant and a preparation method thereof. The aerosol inhalant comprises the following components: active components including umeclidinium bromide and vilanterol triphenyl acetate, a solubilizer, a bacteriostatic agent and a solution carrier. In addition, the invention also discloses a pharmaceutical composition. The active components, namely the umeclidinium bromide and the vilanterol triphenyl acetate, of the aerosol inhalant are good in stability, propellants are not contained, and the prepared aerosol inhalant is small in particle size and beneficial for pulmonary absorption of medicines. The preparation method for the aerosol inhalant is simple in production process, good in repeatability and suitable for industrial production.
Owner:SHANGHAI ANOVENT PHARMA CO LTD
Inhalant device with adjustable inhalation force
ActiveCN111905205AEliminate residueReduce the risk of adverse reactionsInhalatorsInhaled drugEngineering
The invention relates to the field of inhalant devices, in particular to an inhalant device with adjustable inhalation force. The device comprises an inhalation part for inhaling a medicament by a patient, and further comprises a threshold switch with a braking state and an opening state; a threshold of the threshold switch is matched with the value of negative pressure generated in the inhalationpart when the patient inhales air by the inhalation part, and when the threshold switch is in the braking state, the medicament is stopped, so that the patient cannot inhale the medicament by the inhalation part; when the value of the negative pressure generated when the patient inhales the air by the inhalation part is equal to or larger than the threshold, the threshold switch is converted to be in the opening state from the braking state and kept in the opening state, so that the patient can inhale the medicament by the inhalation part; and the threshold of the threshold switch is adjustable. According to the inhalant device, the good medicine supply effect and the good treatment effect can be achieved, the adverse reaction risk of medicine use is greatly reduced, and the inhalant device can be suitable for patients with different physical conditions.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Inhalant used for inhibiting coronavirus, and preparation method and application of inhalant
PendingCN113350323AReduce dosageLow toxicityPowder deliveryOrganic active ingredientsDrugs preparationsCurative effect
The invention relates to an inhalant used for inhibiting coronavirus, and a preparation method and application of the inhalant, and belongs to the technical field of pharmaceutical preparations. The inhalant comprises Remdesivir and / or a pharmaceutically acceptable salt thereof. According to the inhalant used for inhibiting the coronavirus, due to adoption of small-dose pulmonary administration,effective pulmonary administration concentration can be achieved when micronized or aerosolized Remdesivir is inhaled, the coronavirus, especially the novel coronavirus, can be effectively inhibited, the defects of a narrow treatment window and poor safety in the prior art are overcome, a small dosage is given for multiple times, so that the inhalant can be accumulated in the lung so as to form an effective virus inhibition effect, a curative effect can be improved or the dosage of the Remdesivir is reduced, and potential side effects are reduced.
Owner:深圳瑞思普利生物制药有限公司 +1
Pre-metered dry powder inhaler for moisture-sensitive medicaments
The invention is directed to a pre-metered dry powder inhaler provided with a dry powder dose of tiotropium and excipient(s) loaded into a container comprising a dry, high barrier seal, which prevents ingress of moisture so that the fine particle structure of the powder dose is preserved. The dry powder dose has been formed by either volumetric or electric field dose forming methods. The invention is also directed to a dry powder dose of tiotropium loaded into a container as described above. The dry powder inhaler and the dry powder dose of the invention is intended for use in the treatment of asthma and other respiratory disorders.
Owner:BOEHRINGER INGELHEIM INT GMBH
Inhalation device capable of monitoring inhalation amount and used for inhalant device
ActiveCN111956919AActual intake monitoringRealize monitoringMedical devicesInhalatorsInhaled drugPhysical therapy
The invention relates to the field of inhalant devices, in particular to an inhalation device capable of monitoring the inhalation amount and used for an inhalant device. The inhalation device is usedfor being matched with the inhalant device for use. The inhalation device comprises a detection part for displaying the inhaled medicament amount of a patient; and the inhalation device is detachablyconnected with the inhalant device. According to the scheme, the detection part can be of a detection structure formed by combining an existing sensor capable of detecting the medicament amount and adisplayer, and can also be of a structure for displaying the inhaled medicament amount of the patient in other forms. The inhalation device is provided with the detection part for displaying the inhaled medicament amount of the patient, so that the actual inhaled amount of the patient is monitored.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com