Compound dry powder inhalant and application thereof
A dry powder inhaler and compound technology, applied in anti-inflammatory agents, anti-toxic agents, pharmaceutical formulations, etc., can solve the problems of low blood concentration of drugs, poor patient compliance, low drug targeting, etc., to reduce pulmonary fibrosis, Reduces inflammation and oxidative damage and prolongs the effect of residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The preparation embodiment of compound dry powder inhalation
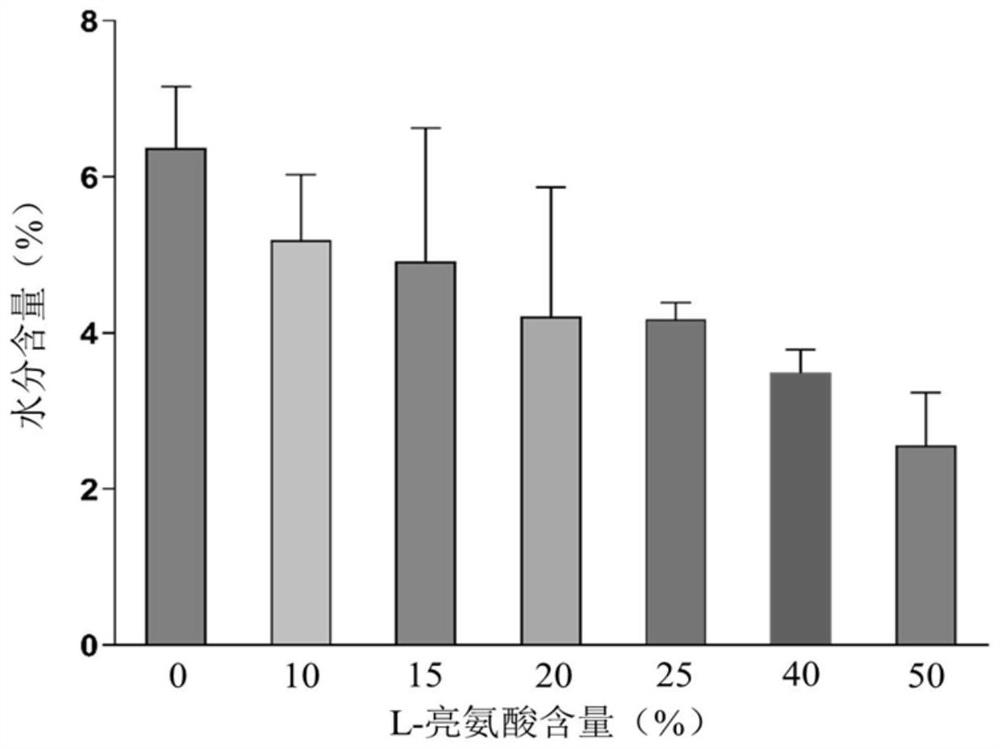
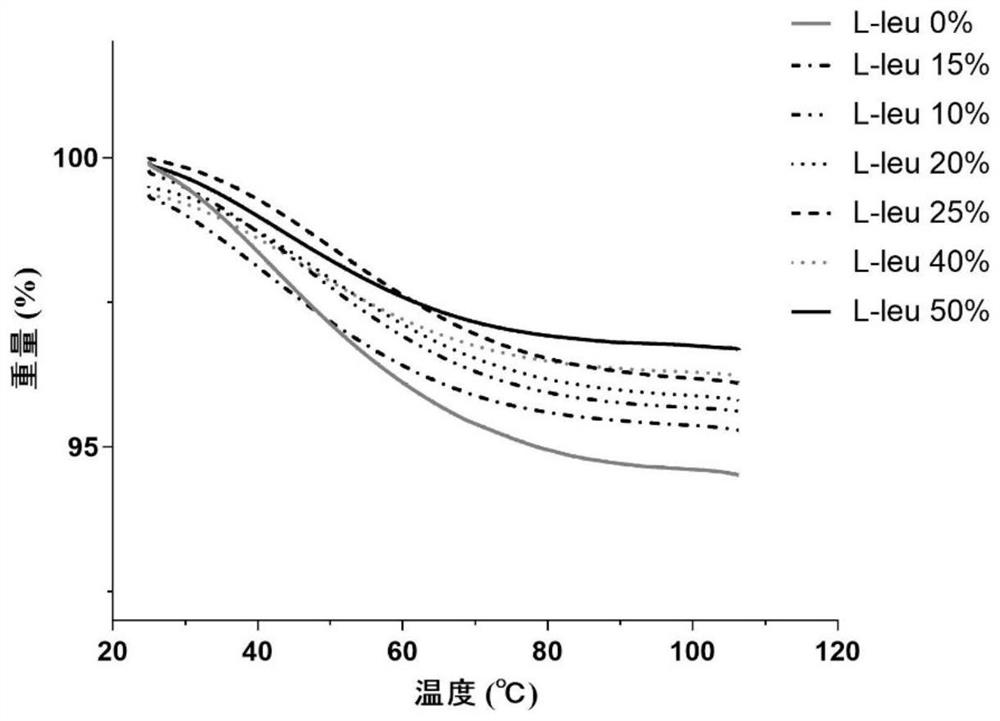
[0054] B-90 nanometer spray dryer is used to produce compound dry powder inhaler. Configure the PBS solutions of baicalin, ambroxol hydrochloride and L-leucine with different mass contents respectively as the feed solution, wherein, in each embodiment, the composition ratio of the feed solution is as shown in the following table 1, each feed The solution is spray-dried under the same parameters: inlet temperature 80°C, air flow 100L min -1 , pump speed 23%, spray rate 60%, internal pressure 34Mbar. After spray drying, the DPI dry powder was collected in a normal temperature drying environment, weighed and sealed, and stored in a normal temperature desiccator.
[0055] Table 1
[0056]
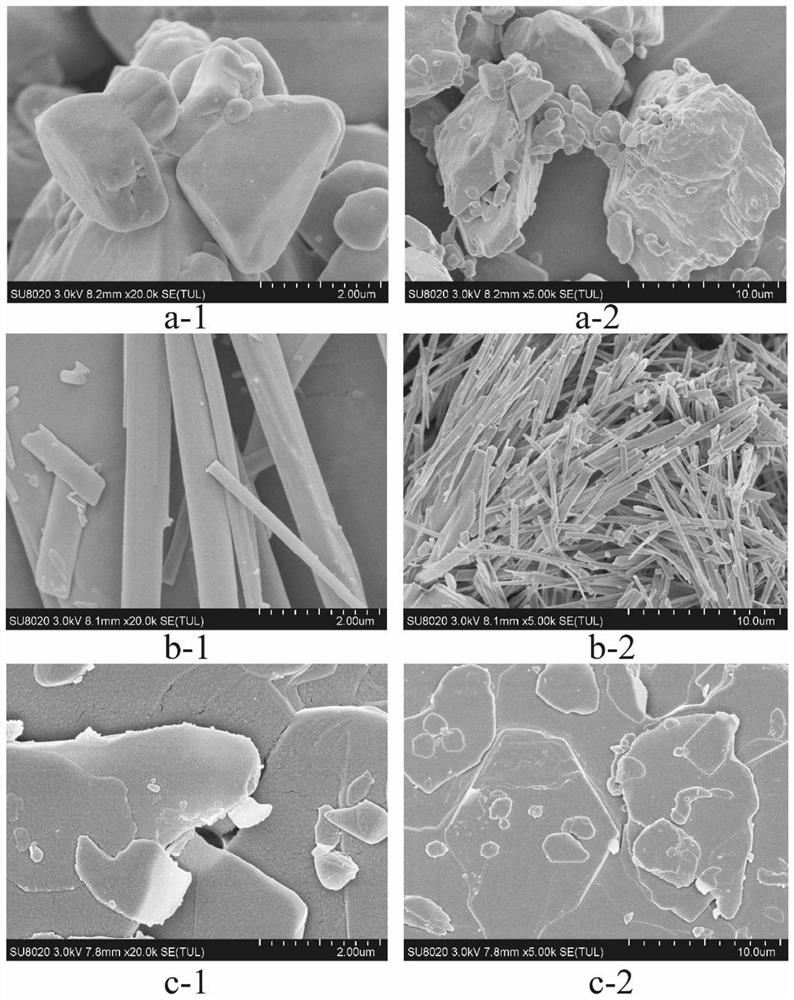
[0057] Quality evaluation of compound dry powder inhaler
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com