Patents
Literature
40 results about "Inhaled drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inhaled drugs, also called “inhalants,” refer to a group of substances that people inhale into their lungs for the purposes of getting high.
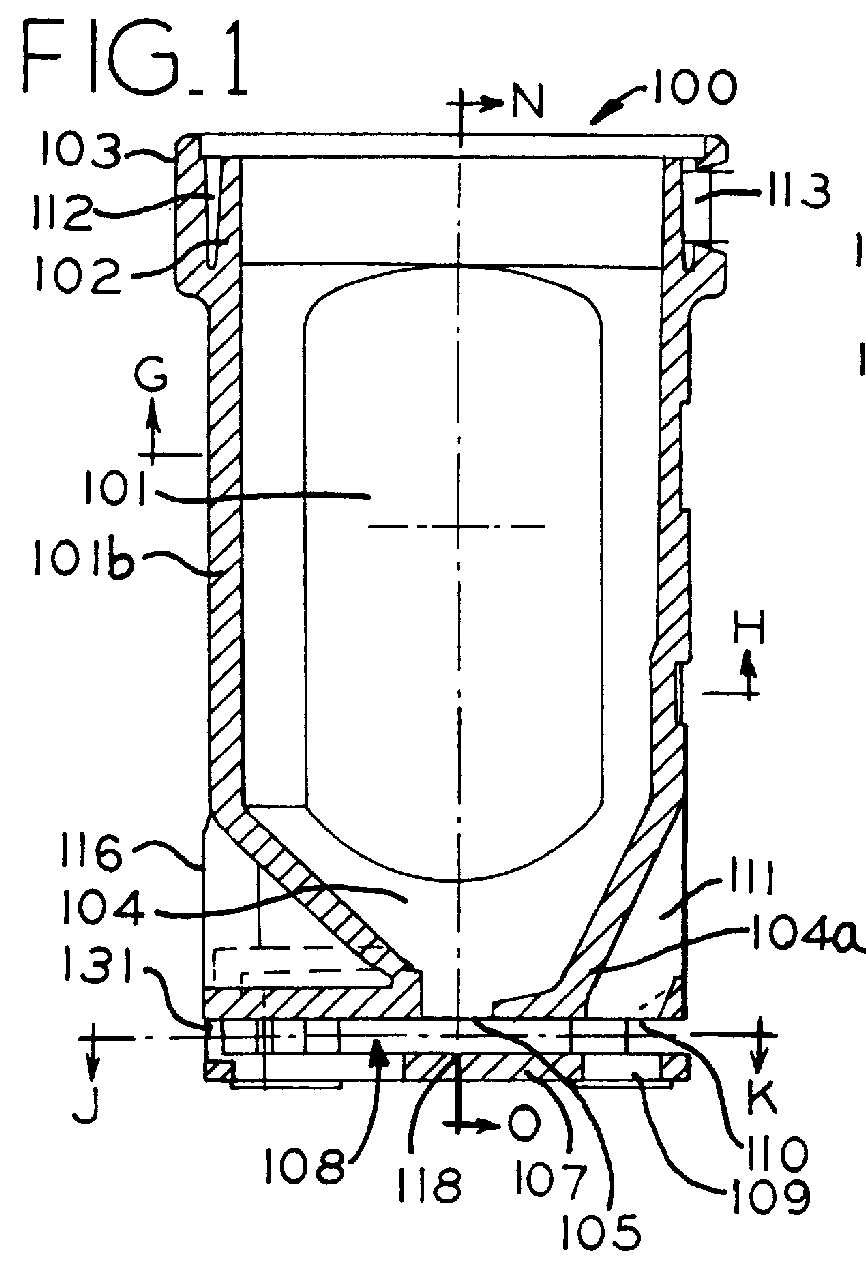
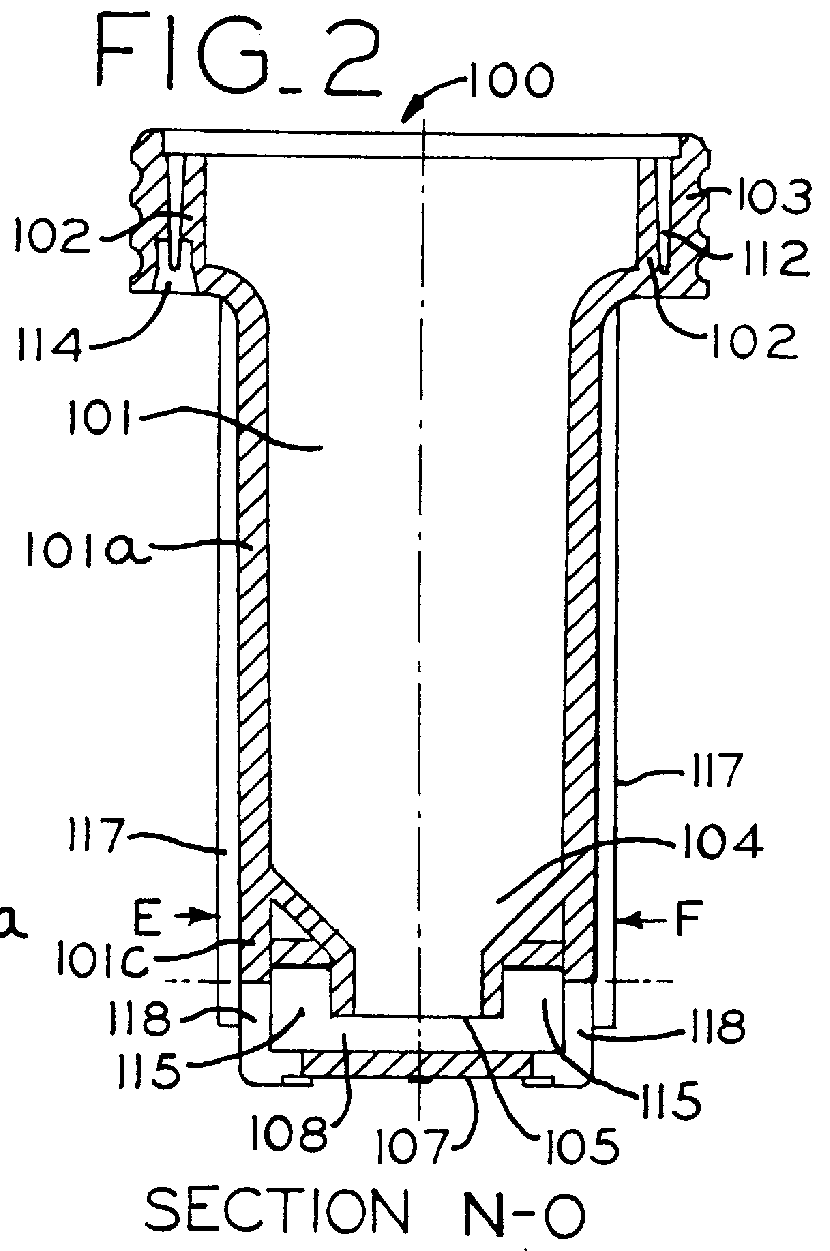
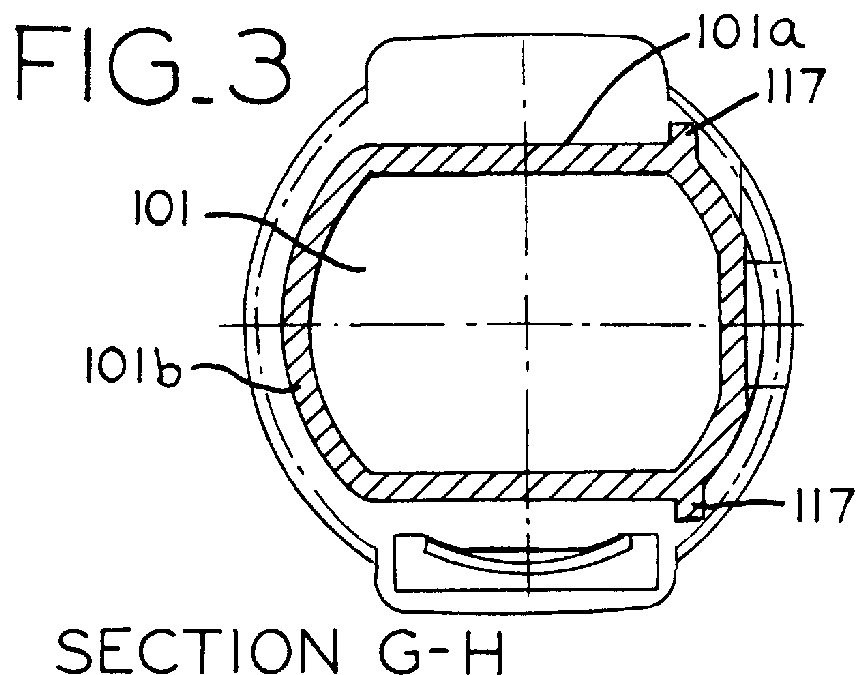
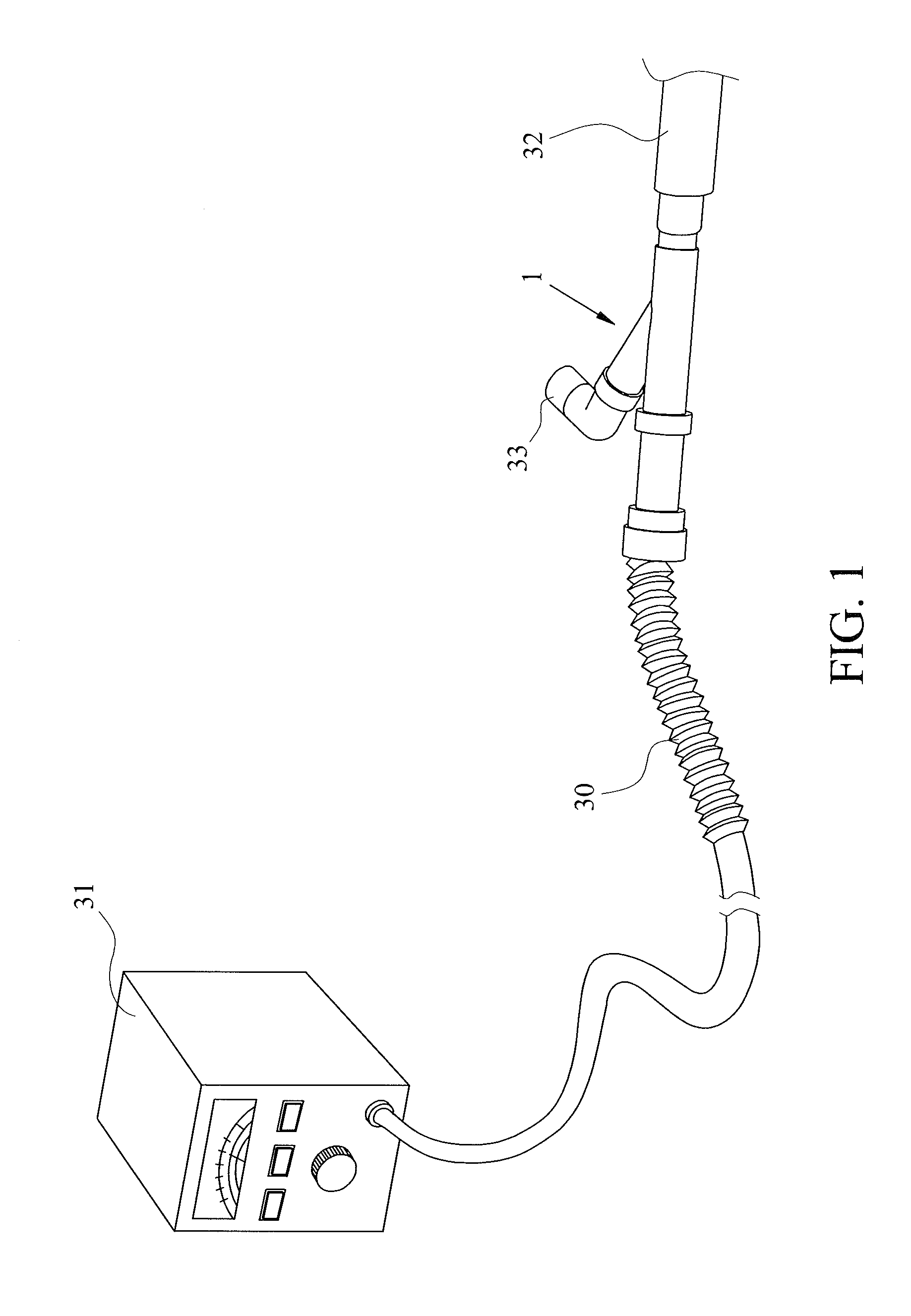
Inhaler for powdered medicaments
InactiveUS6071498ASimple and safe operationSimply and economically manufacturedPowder deliveryAerosol deliveryPowder InhalerInhaled drug
Pharmaceutical powder cartridge for powder inhalers for receiving a medicament depot for a large number of pharmaceutical powder doses, having an integrated metering device which comprises at least one metering cavity for receiving a predetermined quantity of a pharmaceutical powder, the integrated metering device being capable of being moved at least out of a filling position into an emptying position approximately transversely with respect to the flow direction of the pharmaceutical powder, and an inhaler for powdered medicaments, in which inhaler the medicament can be received by a patient by means of an air stream and which has a receptacle for such a pharmaceutical powder cartridge.
Owner:ASTRAZENECA AB
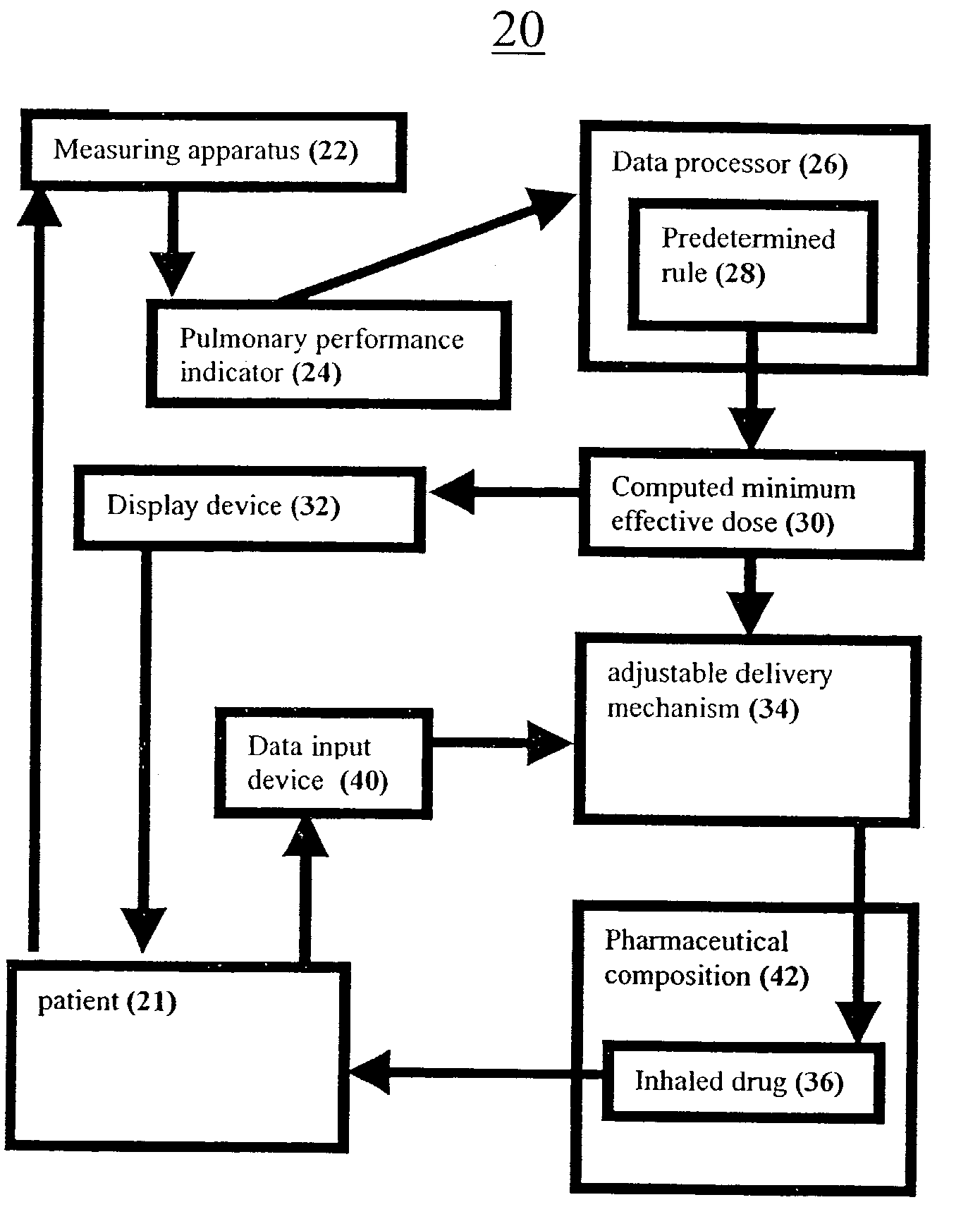
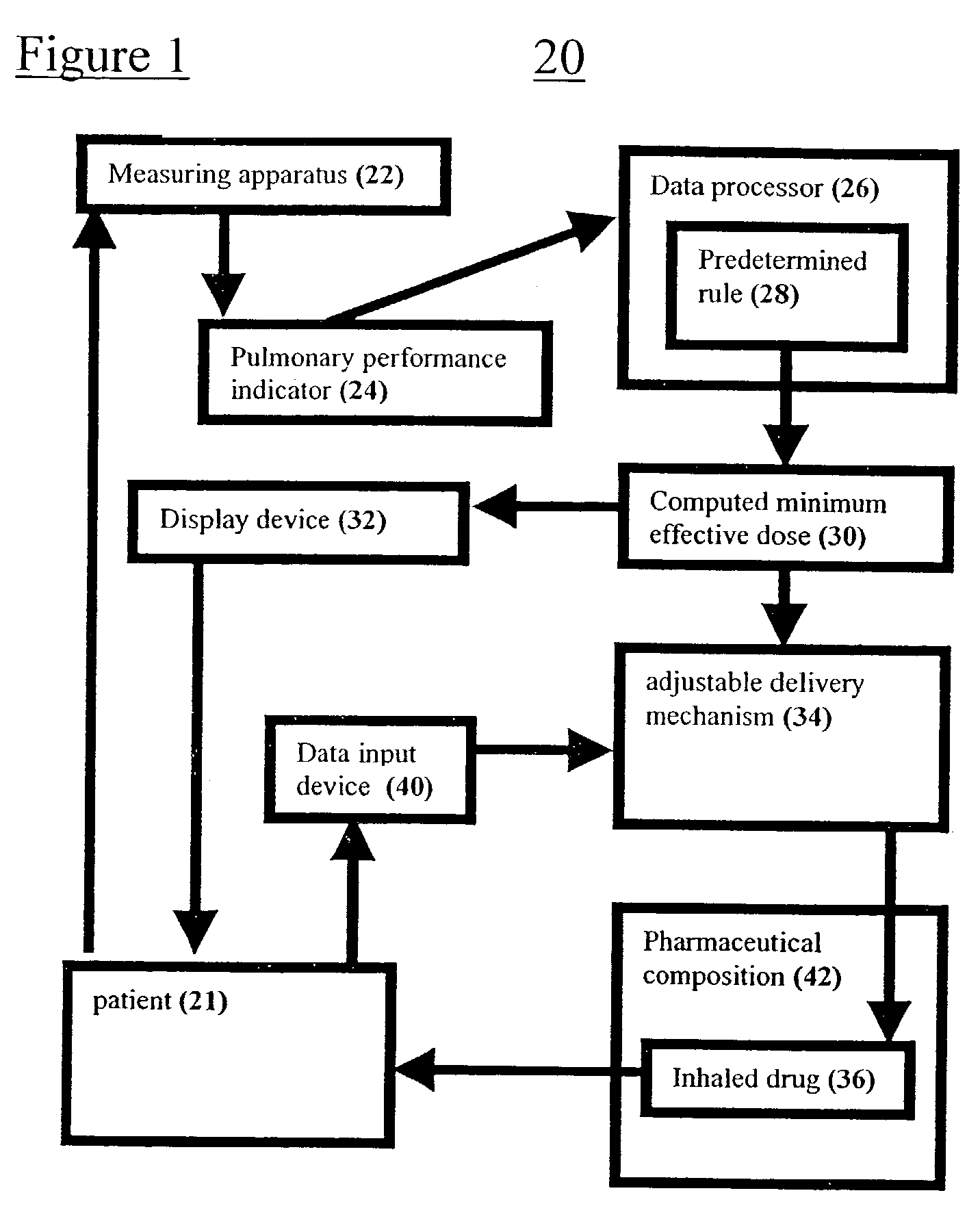
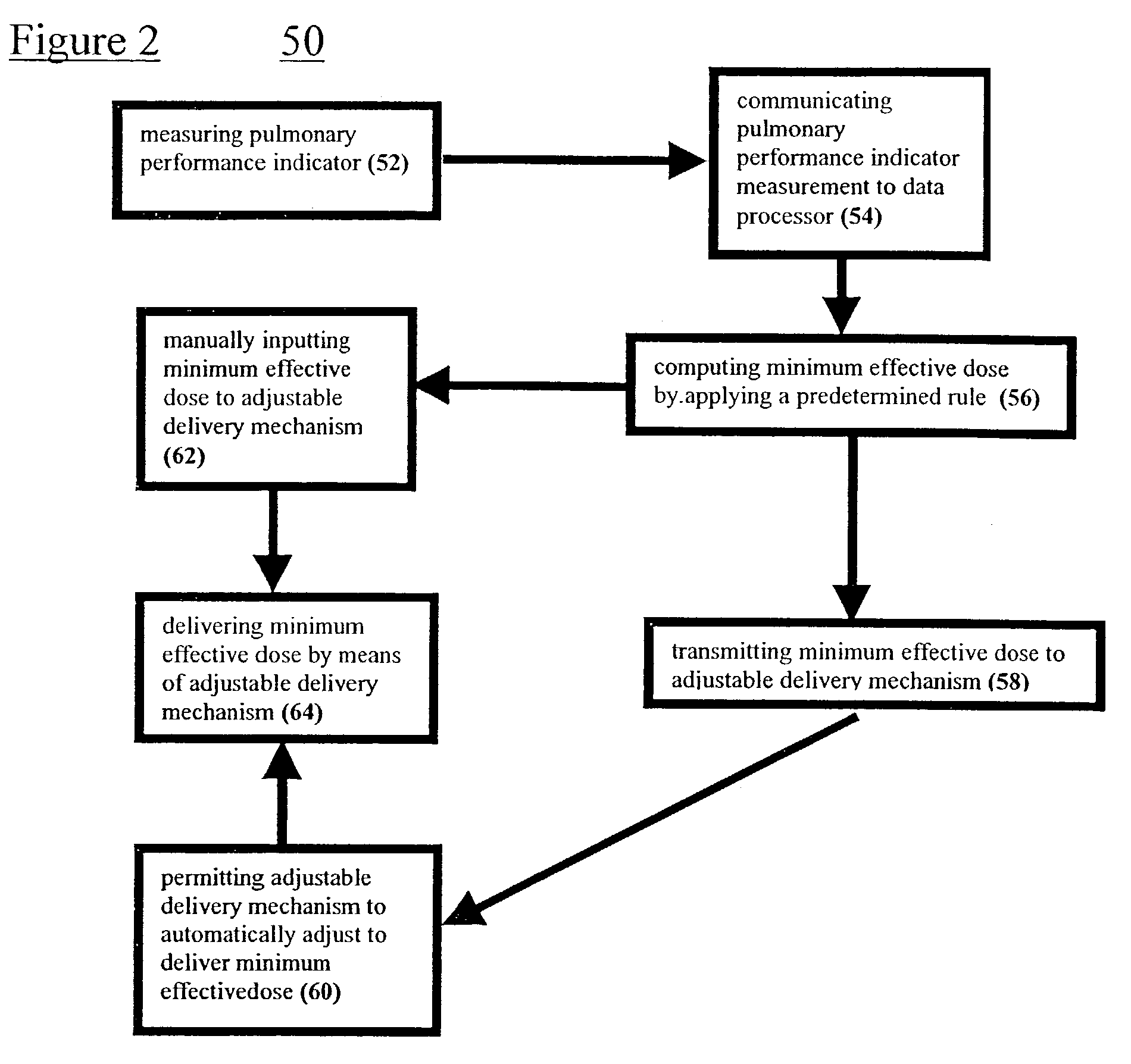
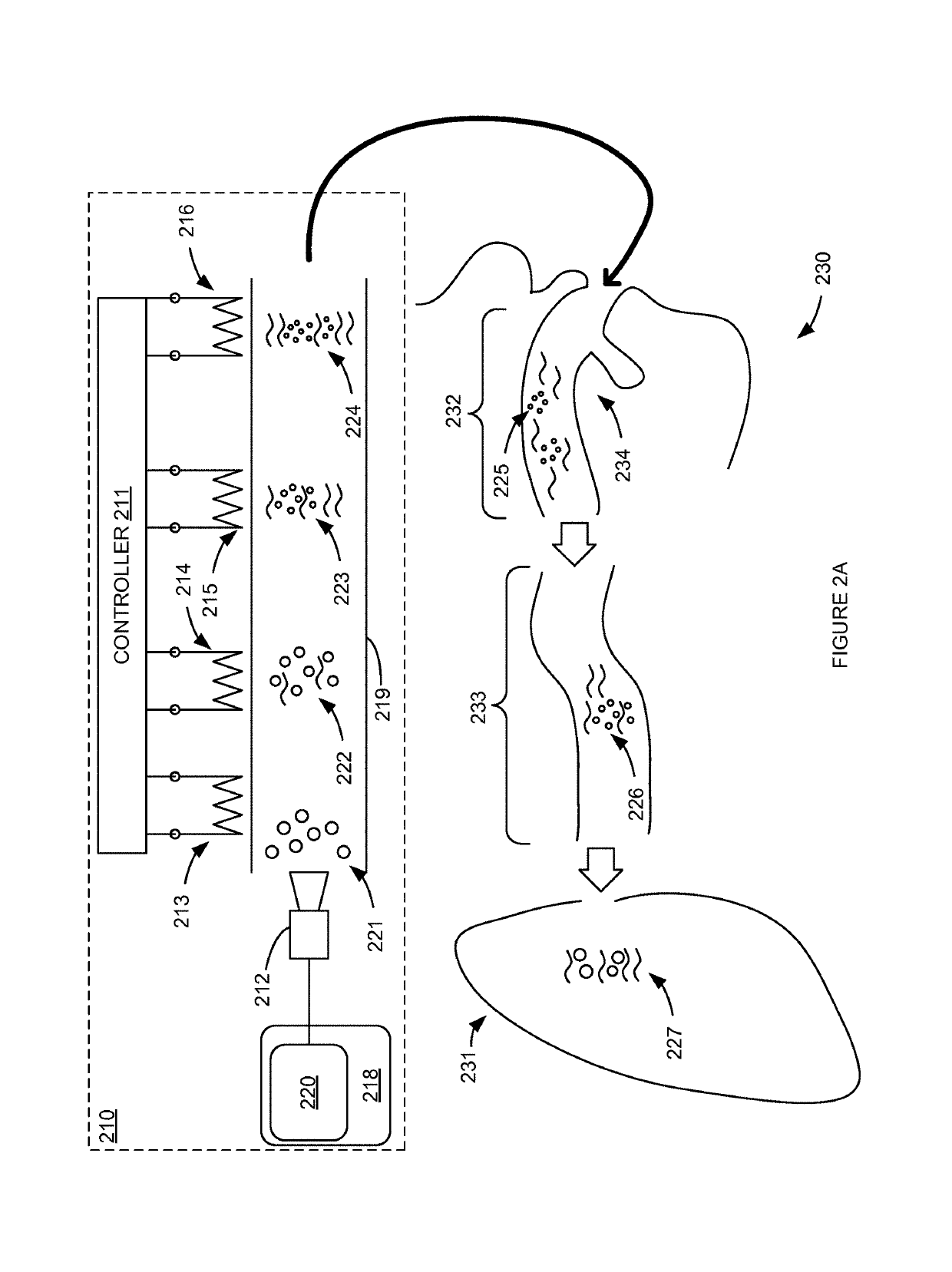
Systems and methods for determining a minimum effective dose of an inhaled drug for an individual patient at a given time
A system and method for determining a minimum effective dose of an inhaled drug for an individual patient at a given time. The system includes an apparatus to measure a performance measurement of the patient and a data processor capable of communication with the apparatus. The data processor provides a computed minimum effective dose for the individual patient by applying a predetermined rule the performance measurement. The method includes measuring a performance measurement of the patient, communicating the performance measurement to a data processor and computing a minimum effective dose for the patient by applying a predetermined rule to the performance measurement within the data processor.
Owner:HUBER ARIE +3
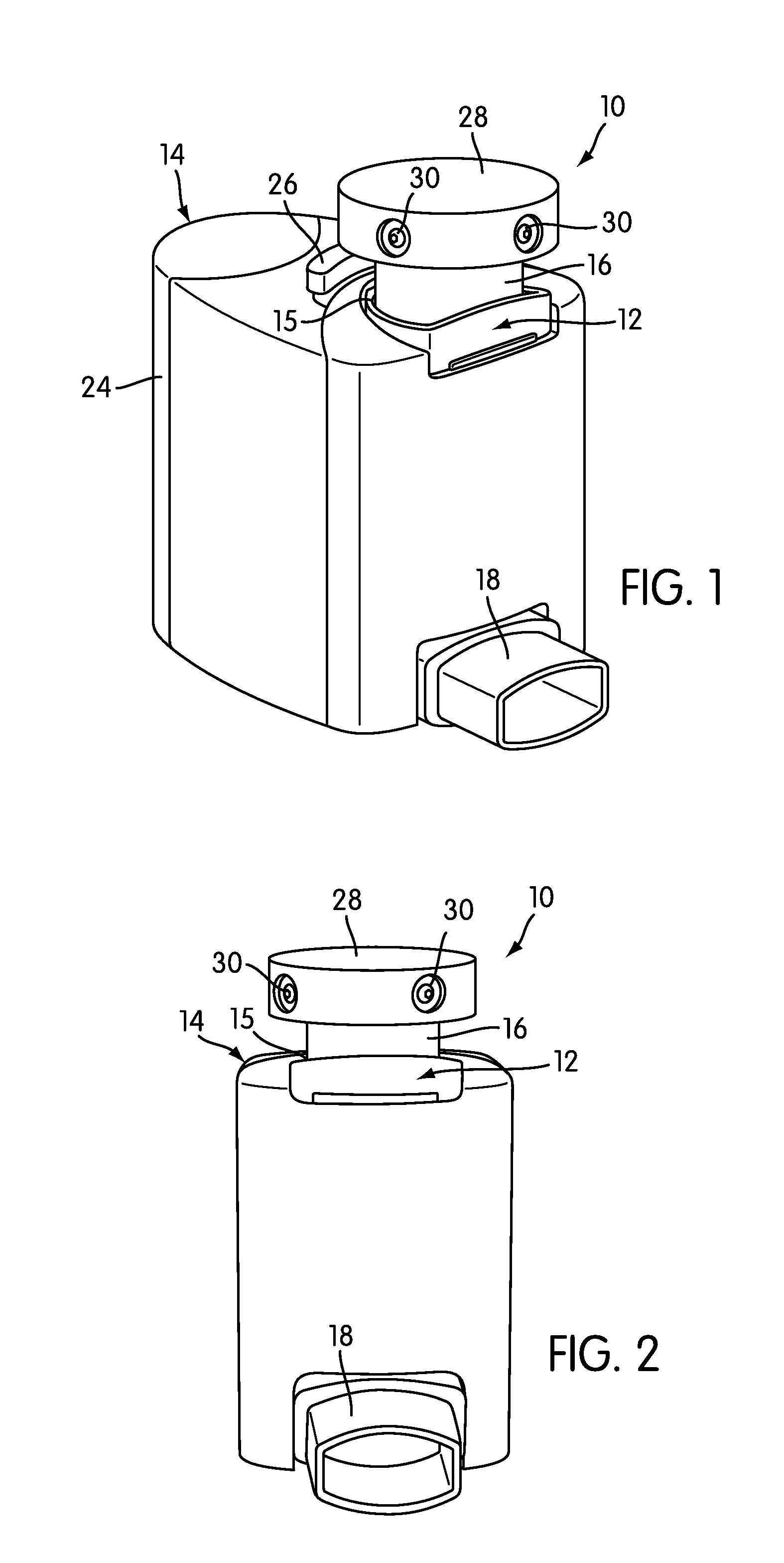
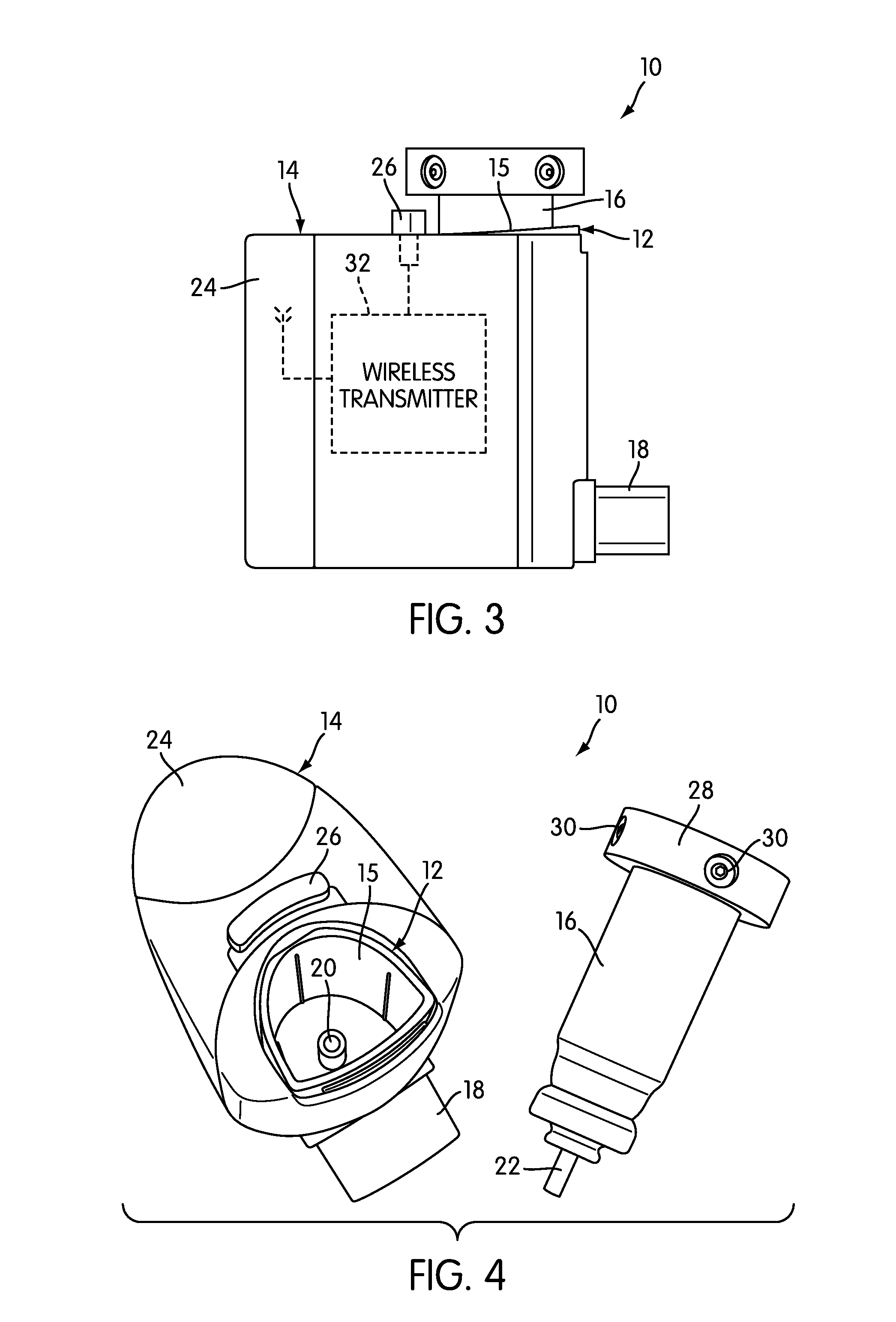
Inhalation device and system for the remote monitoring of drug administration
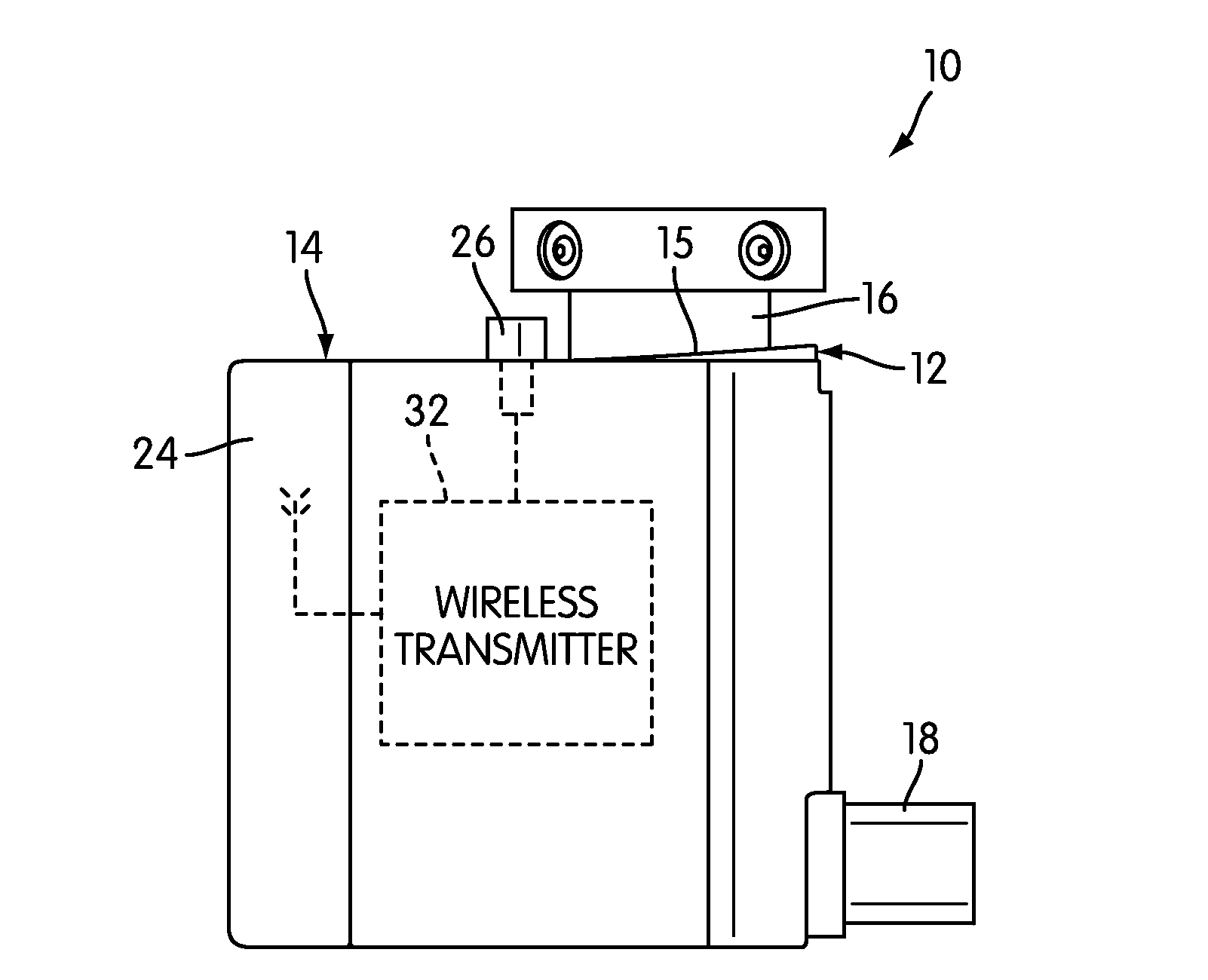
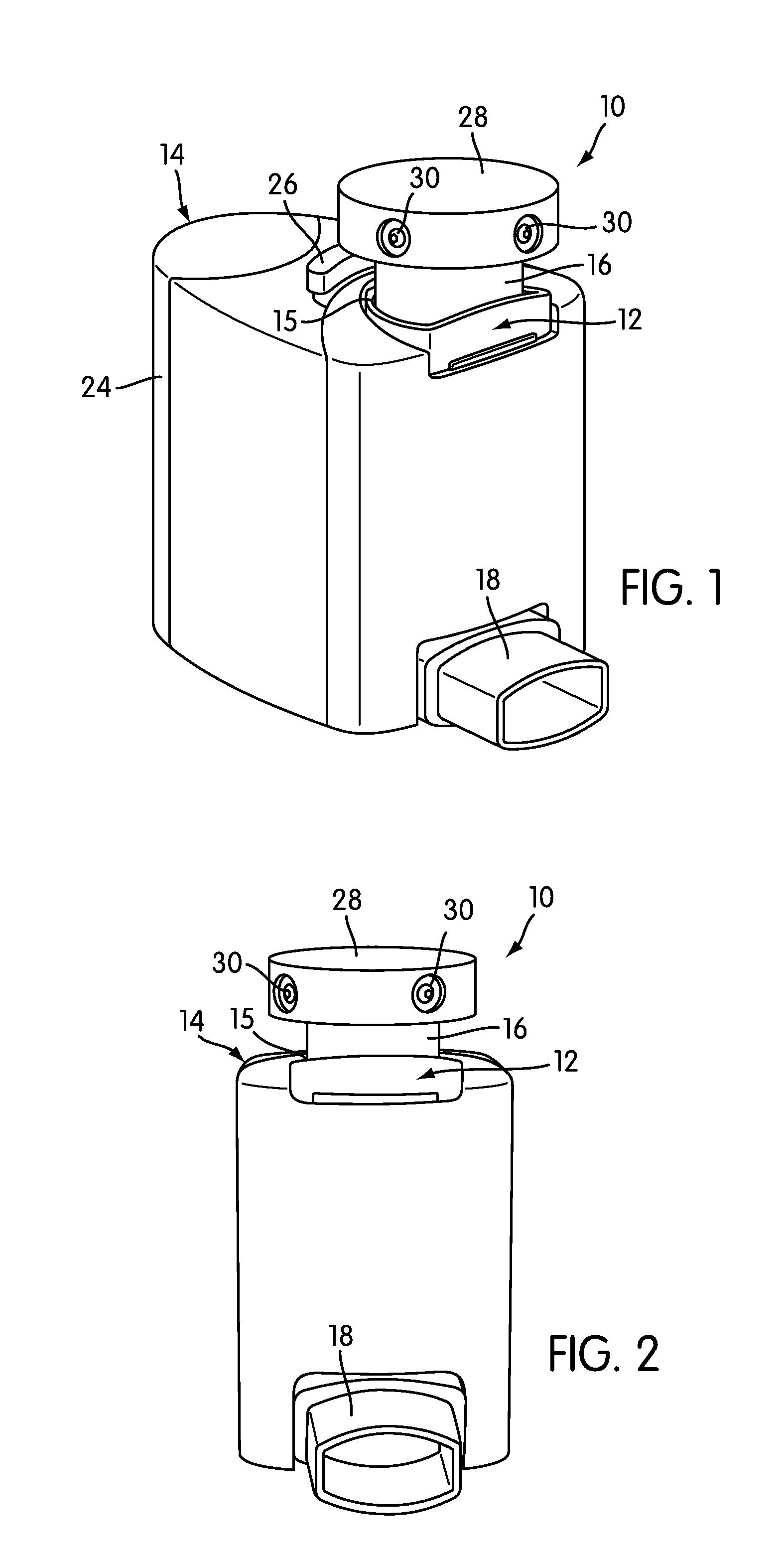
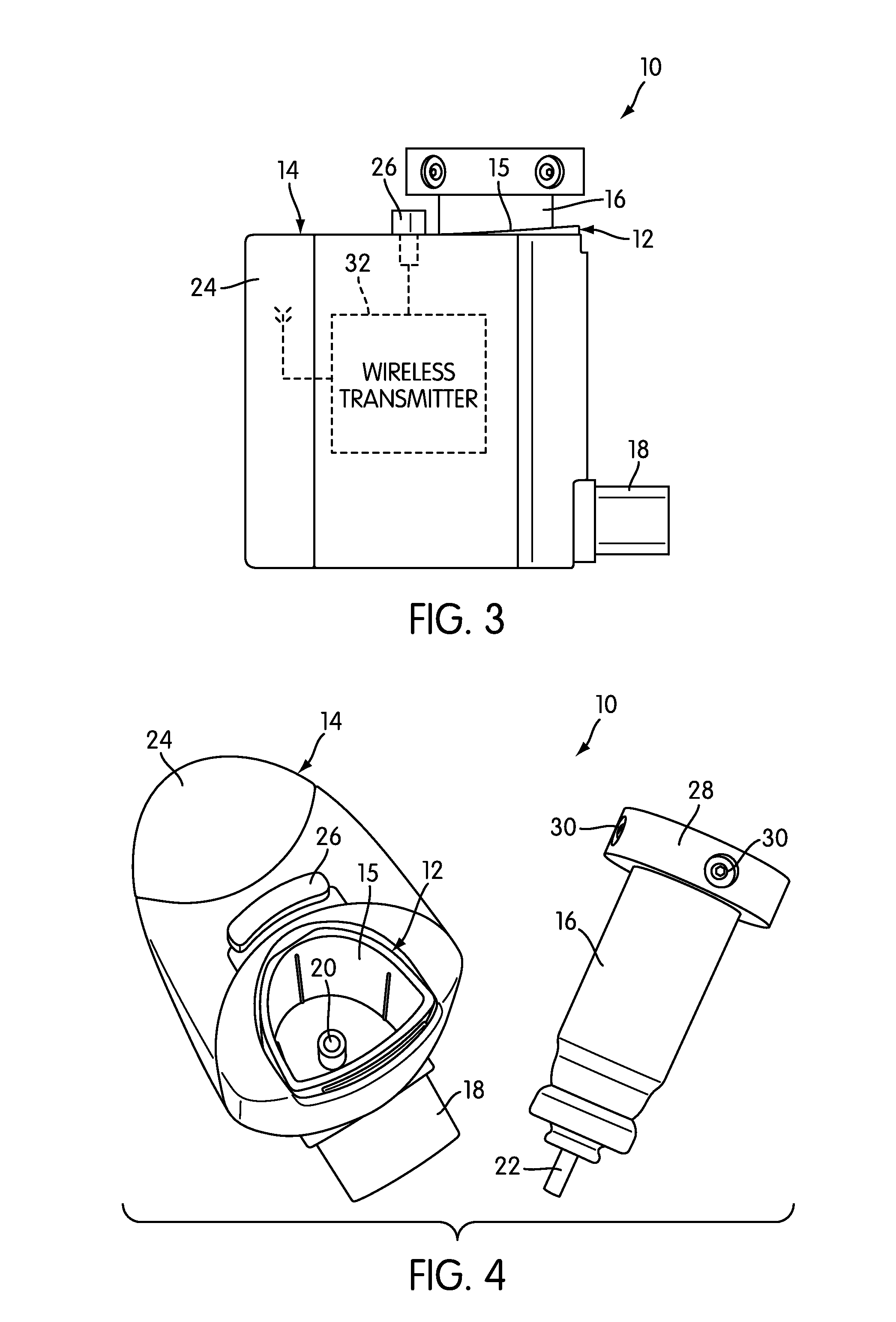
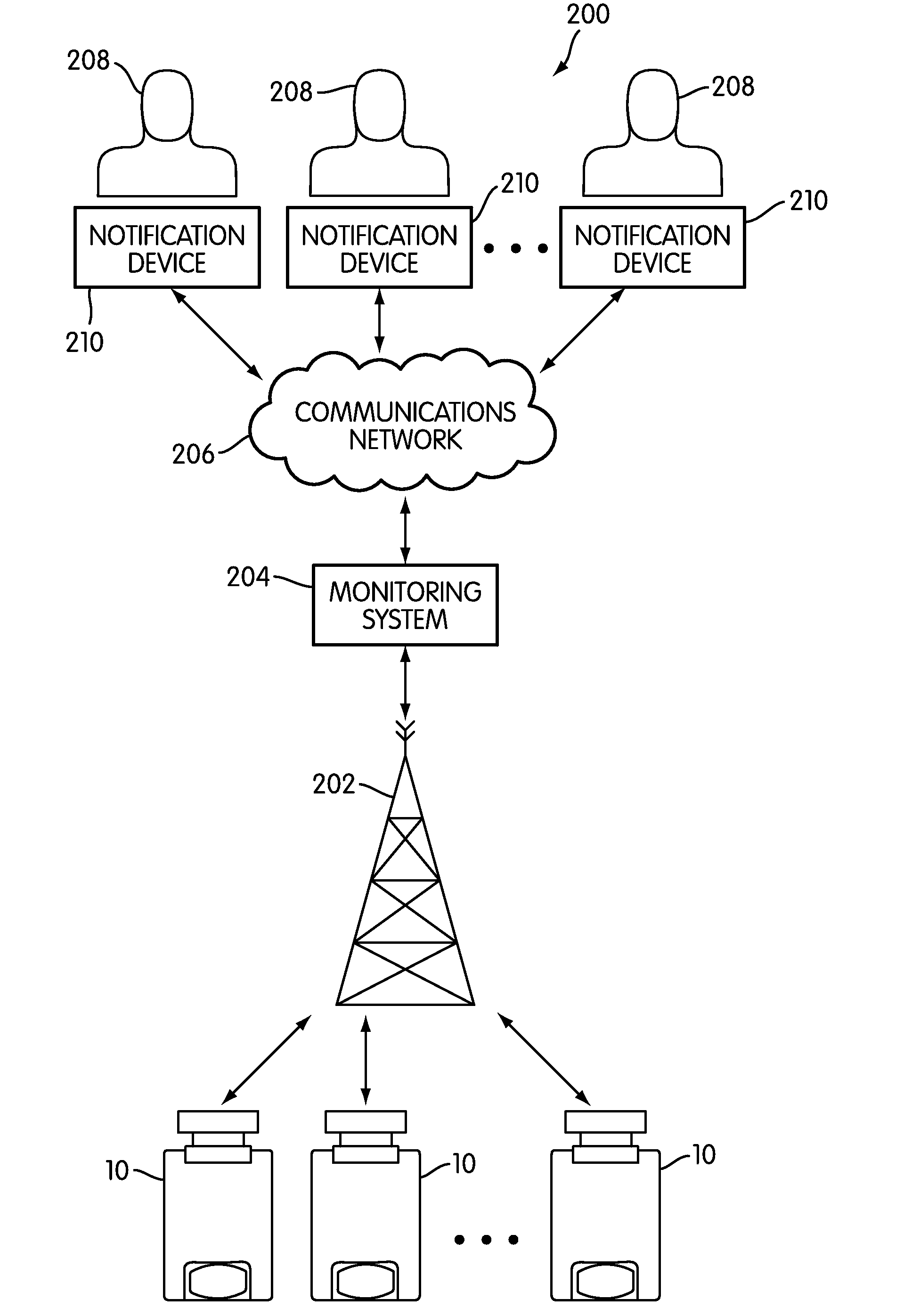
The present invention is directed to a device for monitoring the usage of inhaled drugs by a patient. The device includes an inhaler, a use sensor, a microprocessor, a wireless transmitter and a battery compartment. These components allow information concerning drug usage to be transmitted to health care personnel that can evaluate the data to determine whether there are changes in drug usage characteristics that are indicative of an impending acute attack. The invention includes not only the device, but also the systems and methods in which the device is employed.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
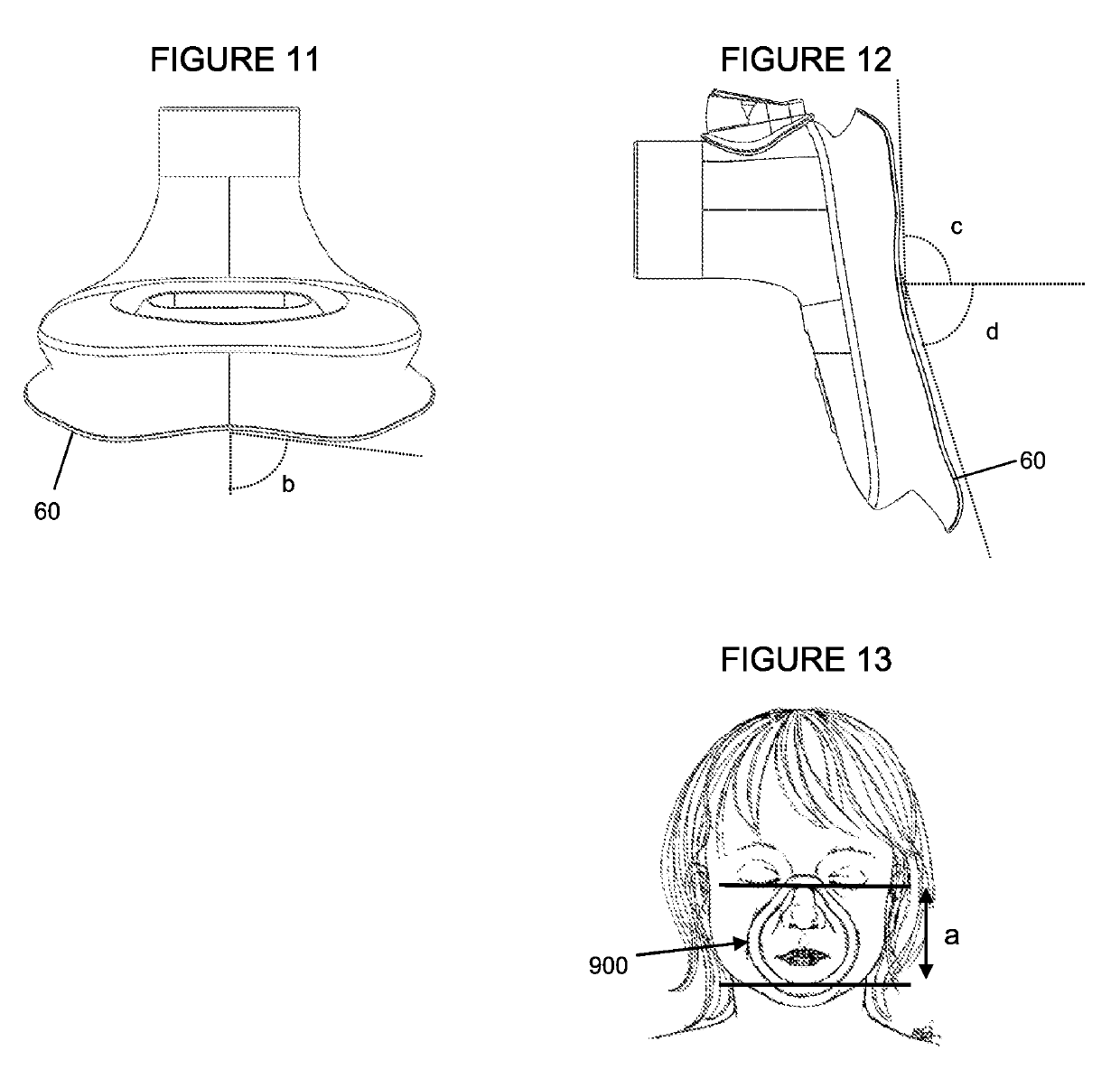
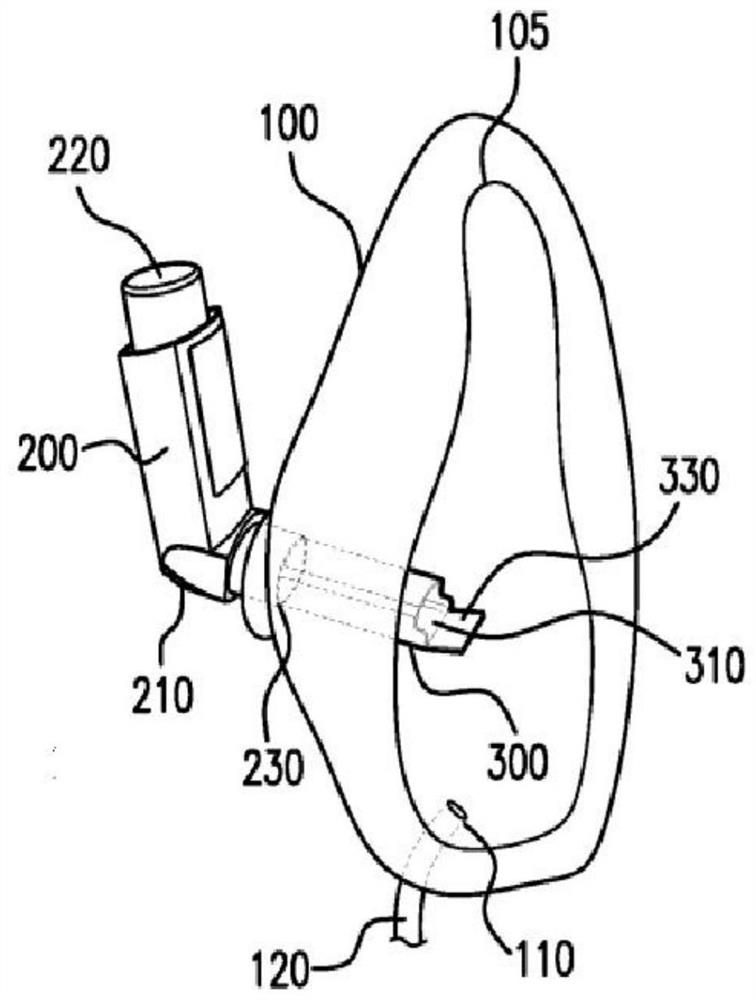
Mask for the administration of inhaled drugs
A breathing mask for use in administering inhalable medications to a patient in need of an inhaled drug is provided. The mask disclosed herein is particularly useful for use with very young children. The mask is made from a flexible molded plastic silicone or elastomeric material, and has an anthropometrically / anatomically / ergonomically contoured shape to provide a good seal, a comfortable fit, and minimal dead space within the mask. The airway is aligned with nose. There may be an orifice for use with a soother device to calm a child using the mask. Also provided is a visual flow indicator to provide an indication of the quality of the seal of the mask on the face.
Owner:NOSTRUM TECH
Instrumented metered-dose inhaler and methods for predicting disease exacerbations
The present invention is directed to devices, systems, and methods for monitoring inhaled drug usage to predict when an acute attack or exacerbation of a disease, such as a respiratory disease, is imminent. Instrumented inhalers that use modular designs with standard components are disclosed, as are systems for monitoring the instrumented inhalers. Also disclosed are methods for determining whether or not a patient's inhaled drug usage pattern indicates that an acute attack or disease exacerbation is imminent, and notifying appropriate medical personnel of any usage patterns indicative of an attack or disease exacerbation. If such an attack or exacerbation is imminent, additional therapeutic agents may be dispensed to the patient or other interventions made.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Instrumented Metered-Dose Inhaler and Methods for Predicting Disease Exacerbations
ActiveUS20100094099A1Easy to addEnhanced couplingRespiratorsDrug and medicationsInhaled drugRespiratory disease
The present invention is directed to devices, systems, and methods for monitoring inhaled drug usage to predict when an acute attack or exacerbation of a disease, such as a respiratory disease, is imminent. Instrumented inhalers that use modular designs with standard components are disclosed, as are systems for monitoring the instrumented inhalers. Also disclosed are methods for determining whether or not a patient's inhaled drug usage pattern indicates that an acute attack or disease exacerbation is imminent, and notifying appropriate medical personnel of any usage patterns indicative of an attack or disease exacerbation. If such an attack or exacerbation is imminent, additional therapeutic agents may be dispensed to the patient or other interventions made.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
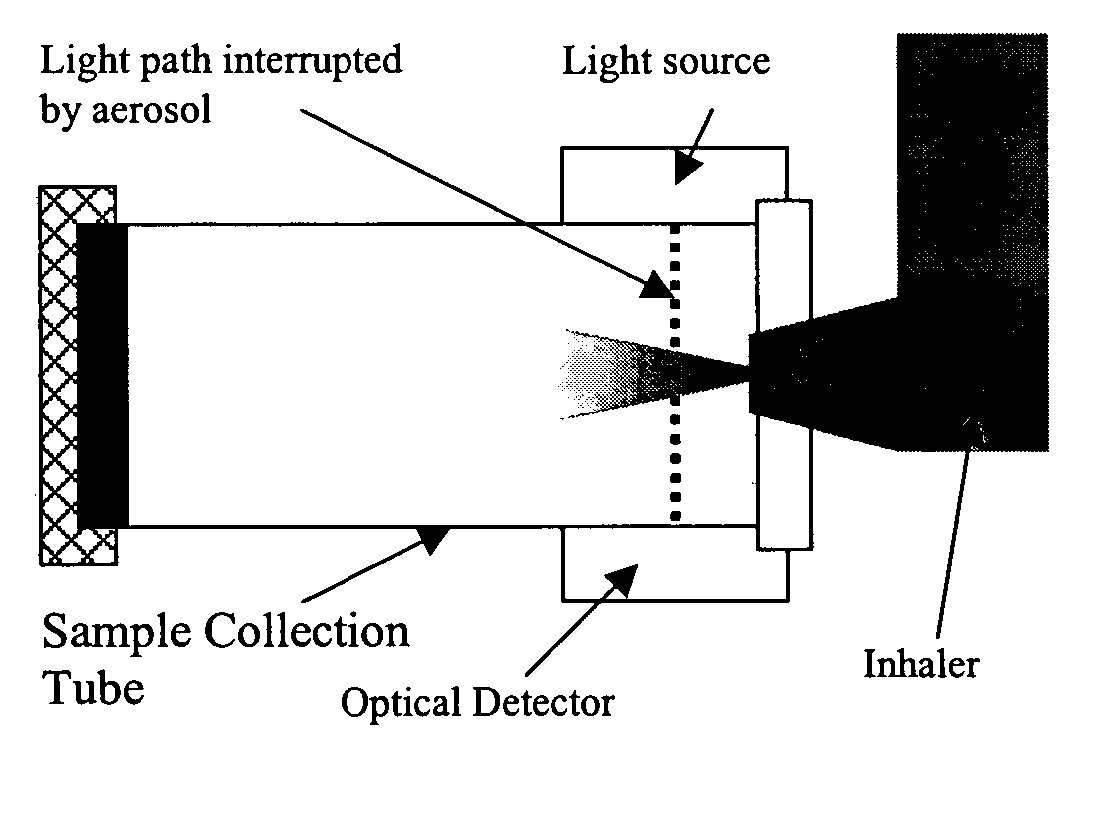
Apparatus, system and method for positive confirmation of inhaled drug delivery by attenuation at point-of-use
InactiveUS20050072421A1Well formedMedication errorMedical devicesMedical atomisersUltrasound attenuationInhaled drug
A system, method and apparatus for providing positive confirmation of inhaled drug delivery by light or radiation attenuation at point-of-use is disclosed, including a tube adapted to be coupled at an outlet to an inhaler; a light or radiation source coupled to the tube; and a light or radiation detector coupled to the tube, where the source transmits light or radiation along a path intersecting a path of an emission such as a drug dose emitted from the inhaler, and where the detector receives the light or radiation from the source.
Owner:NEXT BREATH
Method for determining drug dose for inhaled drug therapy
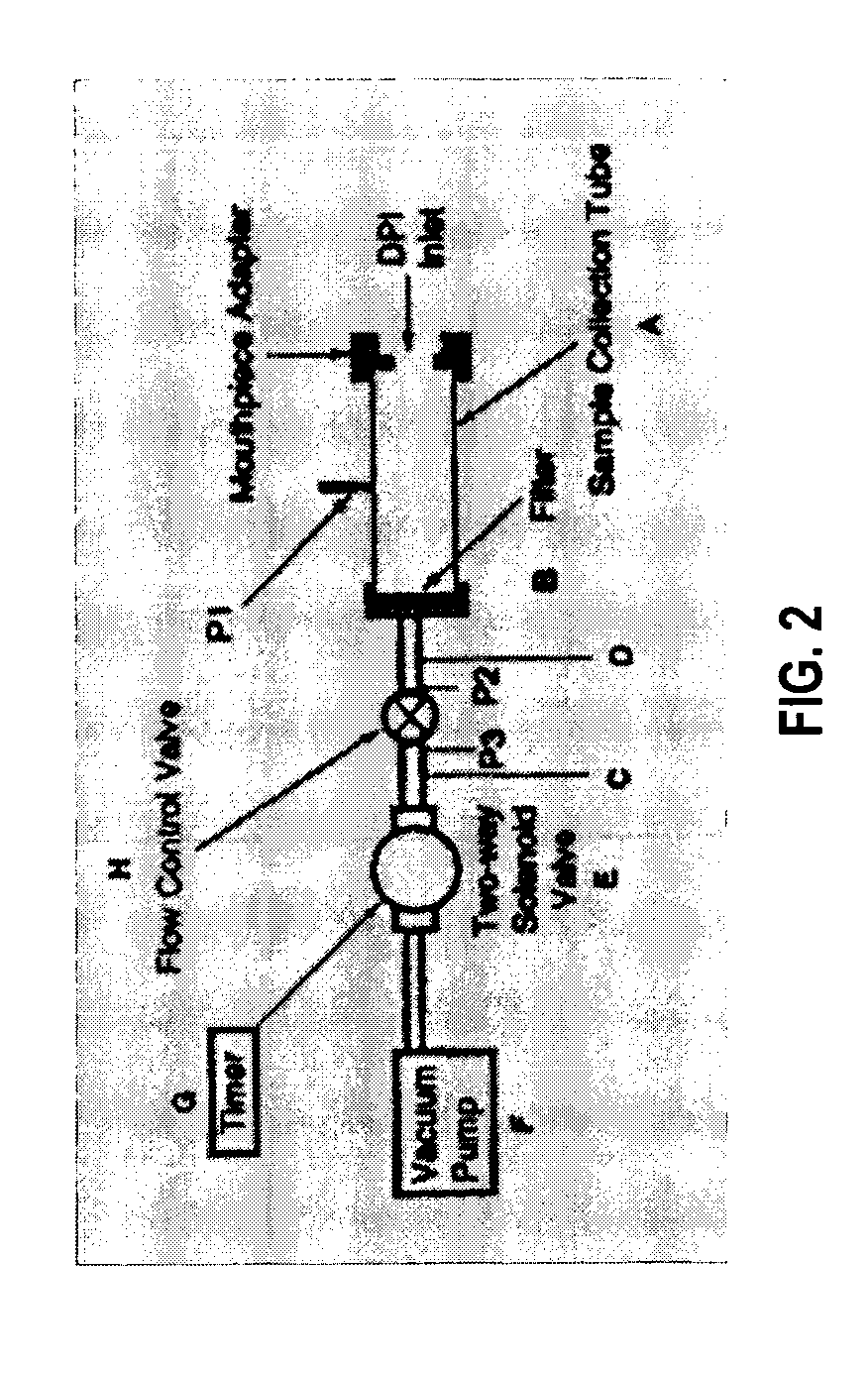
The invention is directed to a method for determining the total amount of liquid medicament containing an active drug substance to be aerosolized and inhaled by a patient in order to deliver a pharmaceutically effective amount (“PEA”) of said drug to the respiratory tract of said patient comprising: A) aerosolizing a measured amount of said medicament liquid containing a known amount of drug (“Aerosolized Dose”) using an aerosolization means connected to said patient via an inhalation tube and an exhalation tube; wherein said exhalation tube contains a filter means and wherein said Aerosolized Dose is less than said PEA of said drug; B) administering said aerosol to the respiratory tract of said patient via said inhalation tube; C) allowing the patient to exhale through said exhalation tube containing said filter wherein any exhaled drug is trapped on said filter; D) measuring the reflectance of the color on the filter media contained in said exhalation filter using a reflectance spectrophotometer to determine the amount of drug on said exhalation filter (“Exhaled Dose”); E) determining the actual dose delivered to said patient (“Delivered Dose”) as follows: Aerosolized Dose minus Trapped Dose minus Exhaled Dose=Delivered Dose, where the Trapped Dose is the amount of drug trapped in the inhalation tube; and F) calculating the total amount of liquid medicament to be aerosolized (“Total Aerosolized Dose”) by multiplying the PEA by the result of dividing the Delivered Dose by the Aerosolized Dose; wherein a solution or suspension of the drug is colored when viewed by the human eye or wherein the drug reacts with a reagent on the filter media of said filter means to produce a color.
Owner:ZIVENA
Thermal modulation of an inhalable medicament
ActiveUS20190314586A1Stop the flowAvoid flowRespiratorsMedical devicesBiomedical engineeringInhaled drug
A thermally modulating inhalable medicament delivery device may deliver an inhalable medicament as an aerosol, vapor, or partial aerosol and partial vapor mixture. The inhalable medicament may be delivered to a target in a subject. Described herein are devices, systems, and methods for delivering an inhalable medicament to a subject.
Owner:BREEDE MICHAEL EDWARD +1
Inhalable drug
A process for preparation of particles of an inhalable drug is described. The process comprises combining a first liquid and a second liquid in a region of high shear, whereby the first liquid and the second liquid interact to form the particles of the drug. One of the first and second liquids comprises the drug or a precursor thereof. In the case where one of the liquids comprises the precursor, the other of the first and second liquids comprises a reagent which reacts with the precursor under high shear conditions to form particles of the drug. In the case where one of the liquids comprises the drug, the other of the first and second liquids comprises a liquid which, when mixed with the liquid containing the drug under high shear, forms particles of the drug.
Owner:NANOMATERIALS TECH PTE
Novel Auxiliary Chamber for Inhaled-Drugs
An auxiliary chamber for inhaled-drugs includes a hollow tube body and a hollow side tube; and a unidirectional air valve which only allows air from an inlet flowing to an outlet end is located at the inlet end of the hollow tube body, wherein the outlet end of the hollow tube body is connected to an endotracheal tube. The hollow side tube connects with a lateral hole of the hollow tube body at one end, and a special universal adapter at the other end. The special universal adapter has a small through hole which is used to accommodate an inhaler therein to release inhaled drugs in the inhaler into the hollow tube body, and the inhaled drugs moves from the inlet end with air flow to the outlet end after entering the hollow tube body to help patients with respiratory failure take medicine.
Owner:HUANG CHUNG CHI +4
Inhalation preparation containing calcitriol and budesonide and preparation method thereof
InactiveCN102247385ALong-term use aloneLower serum IgEOrganic active ingredientsPharmaceutical delivery mechanismDiseaseActive component
The invention discloses an inhalation preparation containing calcitriol and budesonide and a preparation method thereof. The inhalation preparation containing calcitriol and budesonide comprises calcitriol and budesonide as active components and one or more pharmaceutic adjuvants suitable for inhaled drugs, wherein, the weight ratio of calcitriol to budesonide is 100-800:1. The inhalation preparation containing calcitriol and budesonide can be used for treating bronchial diseases in humans or mammals.
Owner:TIANJIN JINYAO GRP
Fog control inhalation device
InactiveCN111450364ASmall weightOverall small sizeMedical atomisersInhalatorsInhaled drugEngineering
The invention relates to a fog control inhalation device. The fog control inhalation device comprises a fog control chamber, an atomization assembly, an adsorption assembly, a communication assembly and a negative pressure generation assembly, wherein a fixed opening and a connection opening are formed in the fog control chamber; the atomization assembly is arranged in the fog control chamber; theatomization assembly comprises a suction nozzle and an inhalation drug channel positioned on the suction nozzle; the suction nozzle is arranged at the fixed opening; the atomization assembly can be used for atomizing a medicine solution and outputting liquid drops formed by atomization to the outer part of the fog control chamber through the suction nozzle; the adsorption assembly is arranged inthe fog control chamber and is used for adsorbing drug particles in inhalation gas which is inhaled into the fog control chamber through the inhalation drug channel; one end of the communication assembly is connected with the connection opening; the negative pressure generation assembly is connected with the other end of the communication assembly; and the negative pressure generation assembly canbe used for driving the inhalation gas in the fog control chamber to flow out from the communication assembly, so that negative pressure is formed in the fog control chamber. A patient can hold the fog control chamber to inhale drugs, so that the convenience and comfort of utilization are improved; the amount of the drugs exhaled by the patient and the dosage of effective drugs which actually enter the body of the patient can be counted and known.
Owner:SHANGHAI SINE YELLOW RIVER PHARMA CO LTD
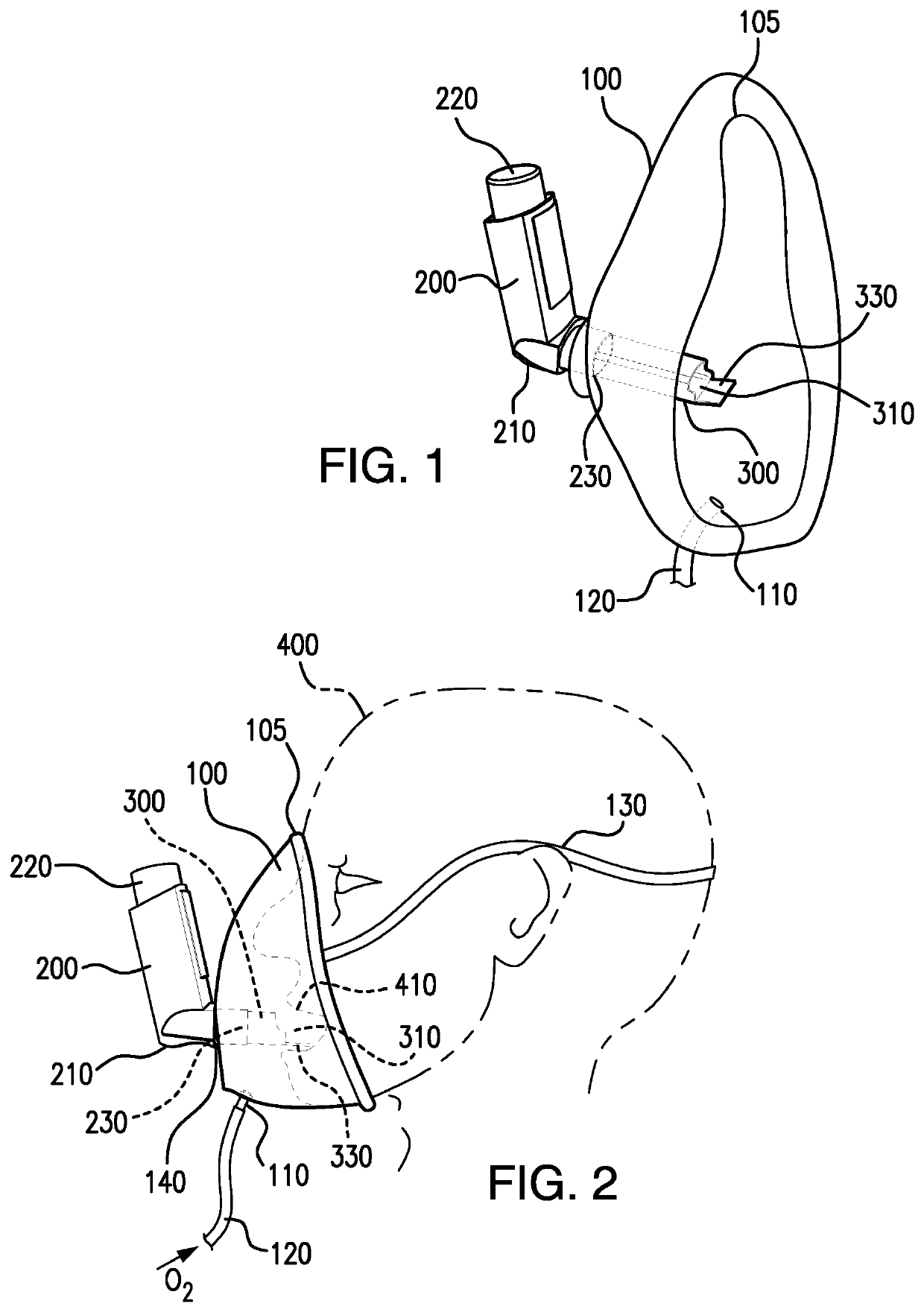
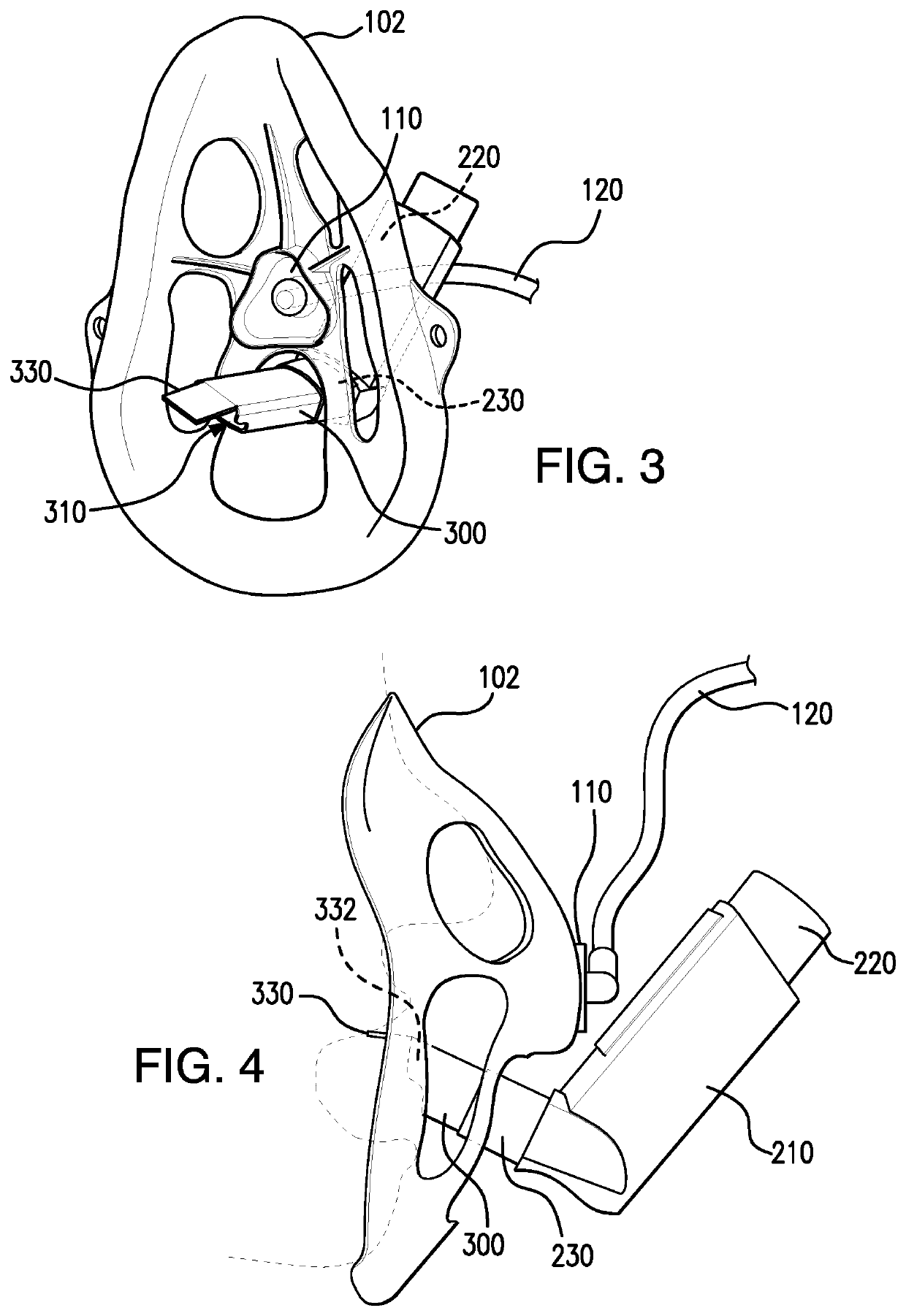
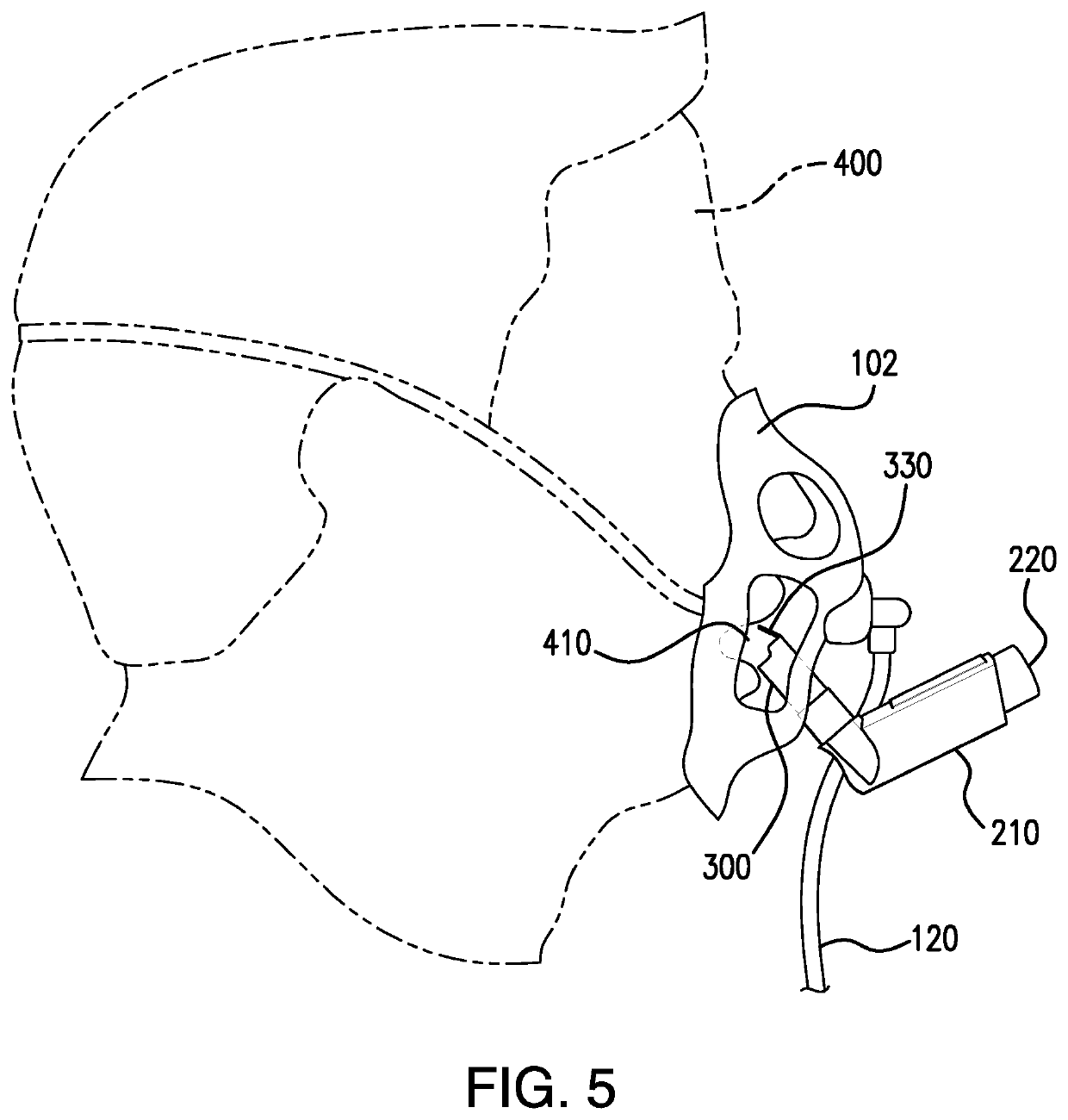
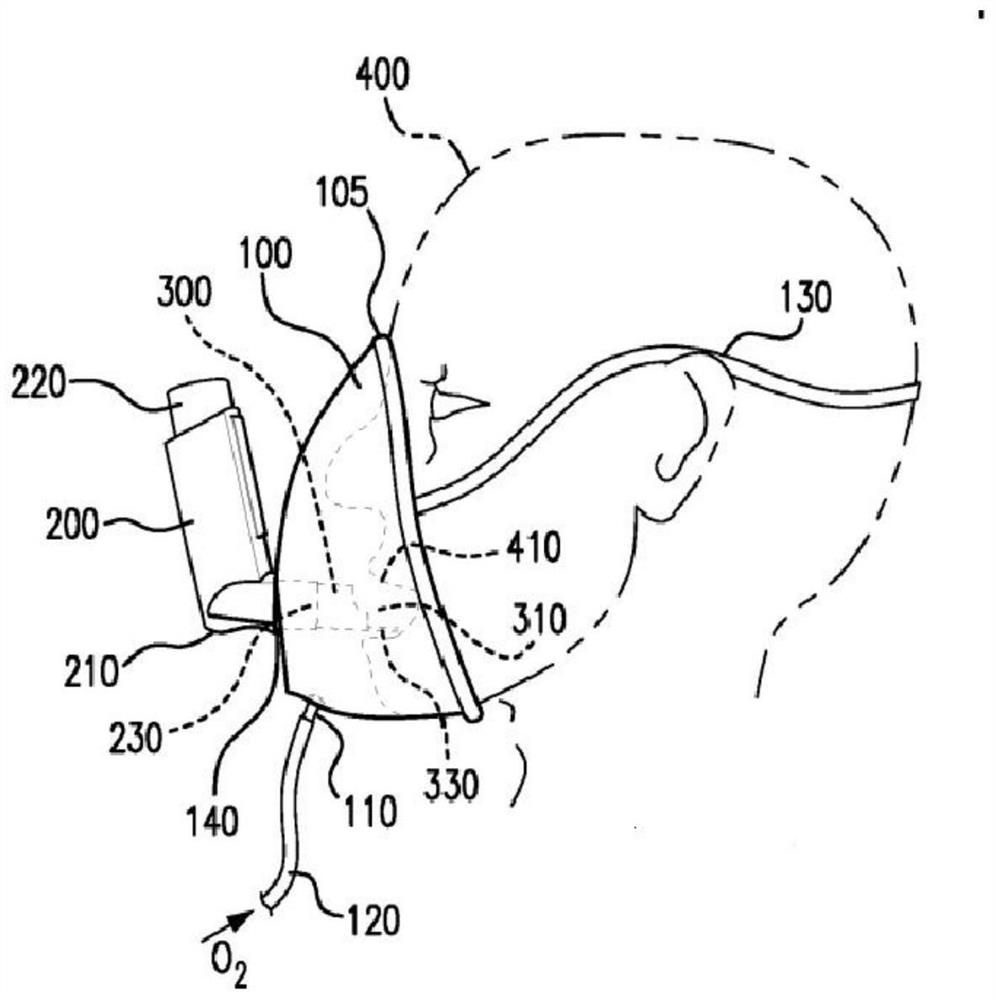
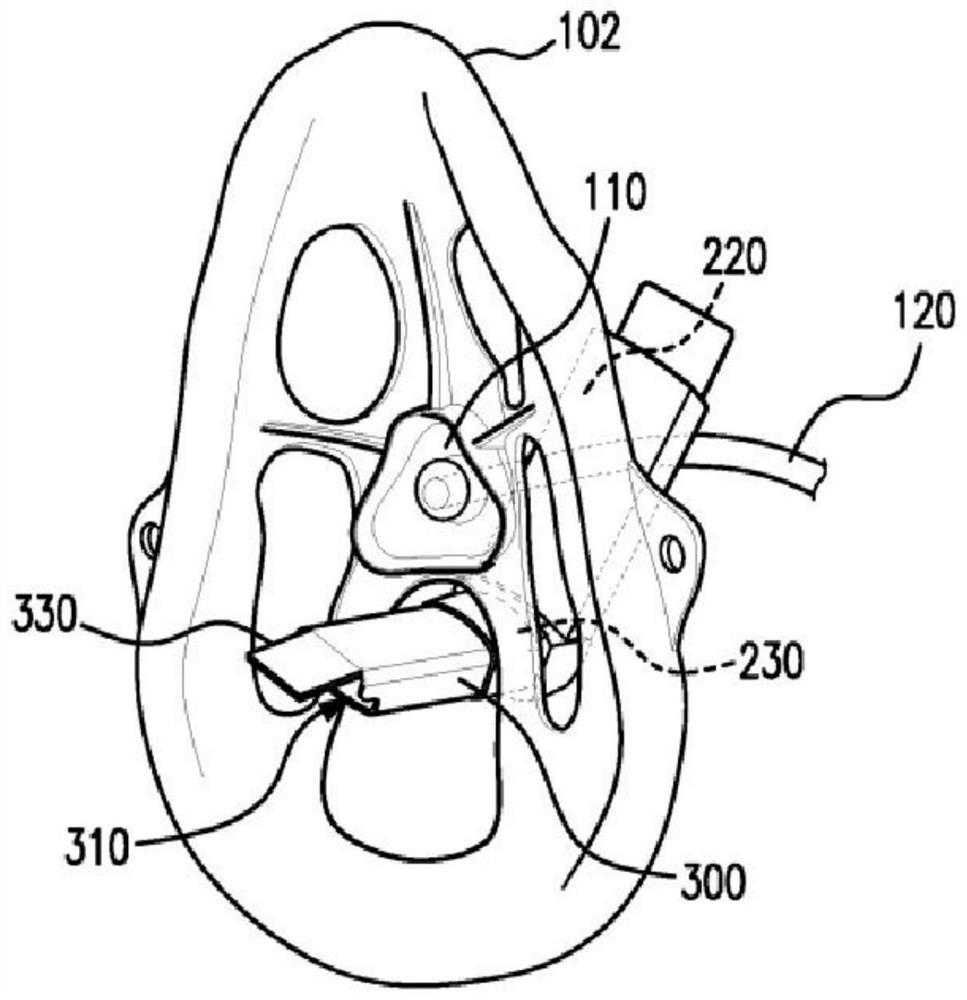
Method and apparatus for simultaneously administering oxygen, and metered dose inhaler medication by inhalation
PendingUS20220347421A1Easy to useEasy to cleanRespiratory masksMedical devicesInhaled drugPharmaceutical drug
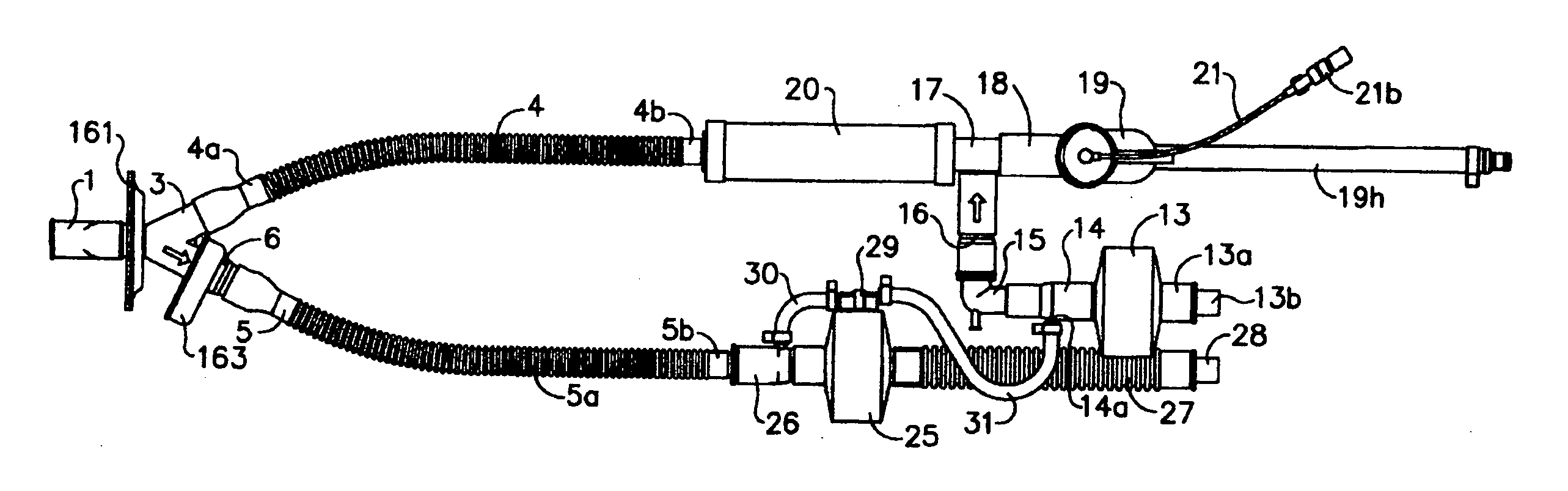
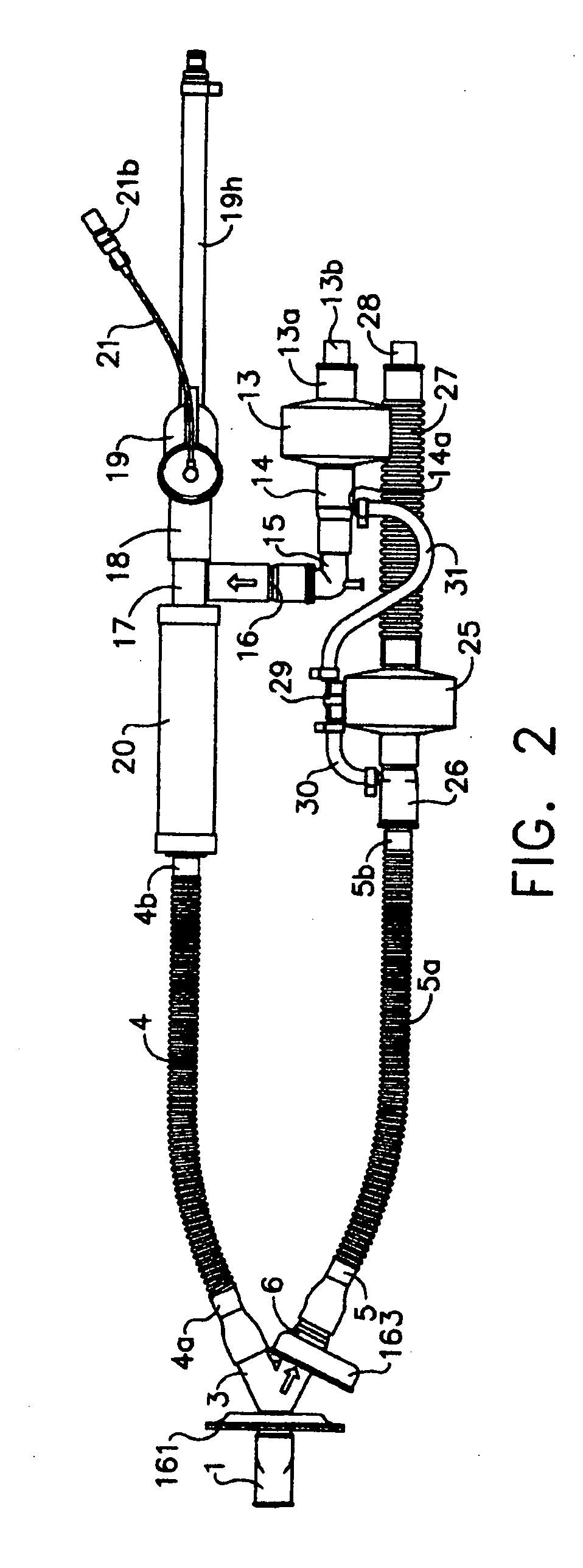
An apparatus and method are provided for administering an inhaled drug to a person while simultaneously administering oxygen from a medical oxygen mask. The inhaled drug is from a pressurized metered dose inhaler (MDI), employing an extender tube about 3-10 cm long that fits into or over the mouthpiece of the inhaler. The MDI with extender tube is inserted into the mask and positioned so that the plume of drug travels through the extender when the MDI is actuated and is directed to just inside the mouth of the person. In an embodiment, an exhalation filter is provided to prevent contamination from infectious agents in the exhaled air from the person.
Owner:GOLDMAN PETER +1
Sevoflurane based tranquilizer
InactiveCN107412209ASedative/hypnotic fastFast fadingNervous disorderPharmaceutical delivery mechanismBronchospasmSide effect
The invention relates to a sevoflurane based tranquilizer which comprises a drug and a drug carrier. The drug refers to sevoflurane. The drug carrier comprises a water absorbing material and a casing; the water absorbing material is used for carrying sevoflurane and the casing is used for covering the water absorbing material for preventing sevoflurane from volatilizing; the casing is provided with a slit, the slit is provided with a sealing unit, and sevoflurane volatilizes from the slit during treatment with the tranquilizer. The sevoflurane based tranquilizer is an inhaled drug for people to take in a breathing way, has quick action for reducing stress or tension, has low side effect, is extincted rapidly after taking effect, and has no influence on daily life and work of people taking the tranquilizer. Besides, the sevoflurane based tranquilizer is free of pungent smel and avoids tracheospasm or bronchospasm, and can be applied to sleeping induction for children.
Owner:SICHUAN CANCER HOSPITAL
Mask for administration of inhaled medication
A breathing mask for use in administering inhalable medications to a patient in need of an inhaled drug is provided. The mask disclosed herein is particularly useful for use with very young children. The mask is made from a flexible molded plastic silicone or elastomeric material, and has an anthropometrically / anatomically / ergonomically contoured shape to provide a good seal, a comfortable fit, and minimal dead space within the mask. The airway is aligned with nose. There may be an orifice for use with a soother device to calm a child using the mask. Also provided is a visual flow indicator to provide an indication of the quality of the seal of the mask on the face.
Owner:INSPIRX
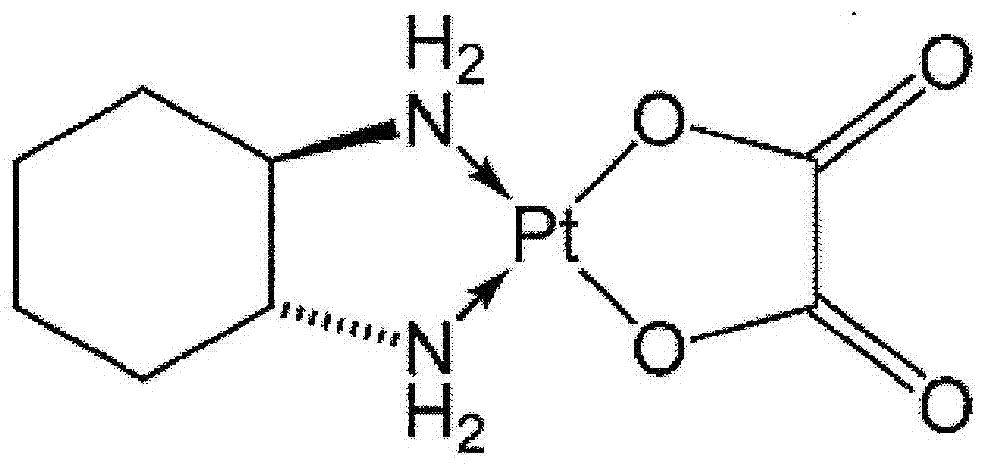
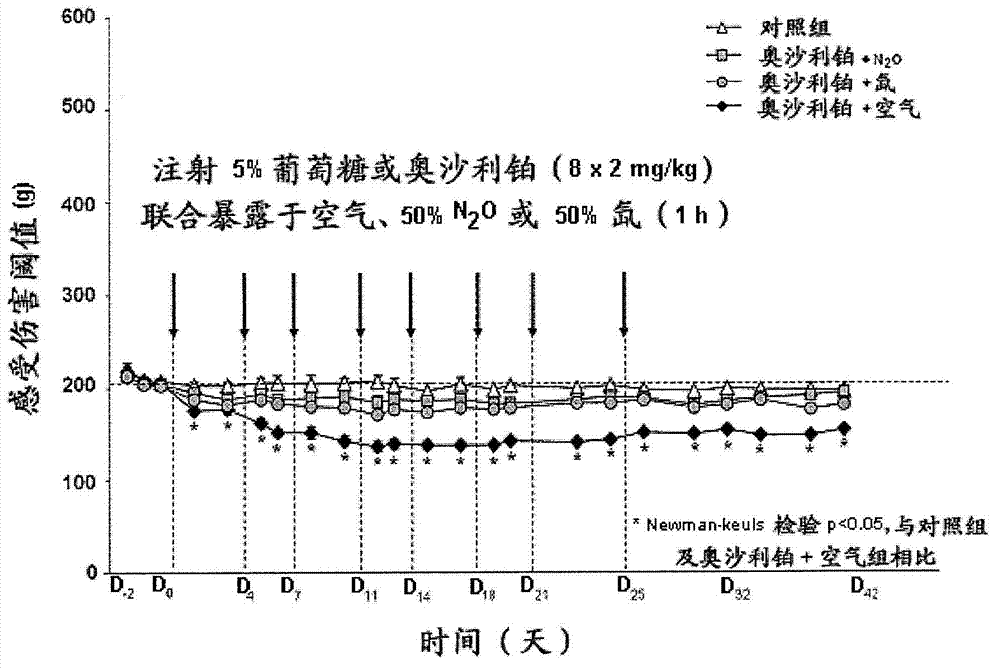
Use of inhaled nitrous oxide or xenon for preventing neuropathic pain induced by cancer chemotherapy
ActiveCN103040859ALower latencyHeavy metal active ingredientsOrganic active ingredientsPlatinum saltsInhaled drug
The invention relates to a gaseous inhalable medicament containing xenon or N2O as active ingredient for use by inhalation for preventing and / or for treating neuropathic pain or pains caused by at least one cancer chemotherapy substance administered to a patient suffering from cancer, in particular a patient suffering from breast cancer, lung cancer, ovarian cancer, prostate cancer, colon cancer, rectal cancer or a gastric cancer or cancer of the upper aerodigestive tracts. The cancer chemotherapy substance contains one or more compounds chosen from platinum salts, taxanes, alkaloids, thalidomide and bortezomib, in particular paclitaxel, docetaxel or oxaliplatin. The effective volume proportion of nitrous oxide or of xenon is between 5 and 70%.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
Inhalant device favorable for inhalant to enter deep part of lung
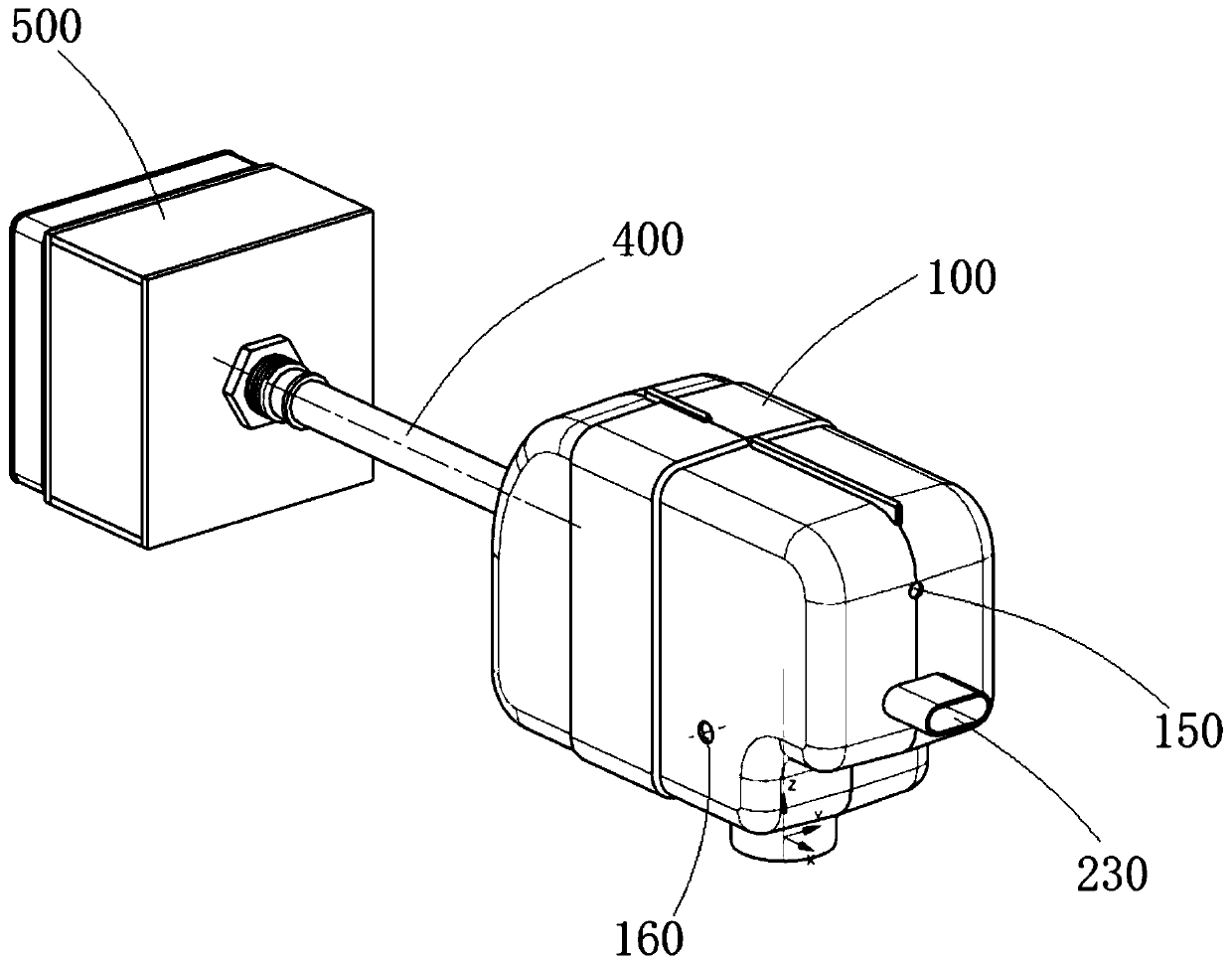
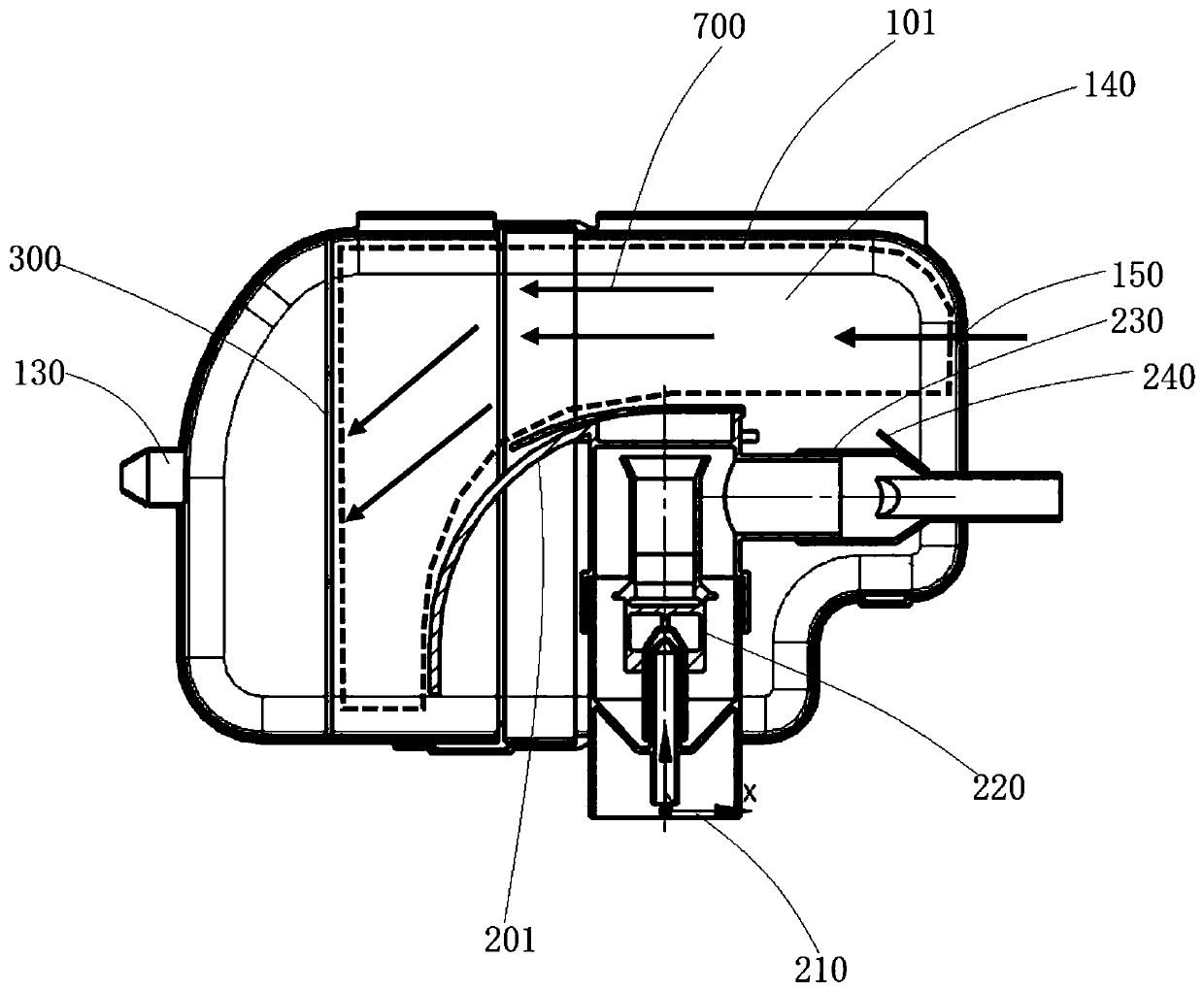
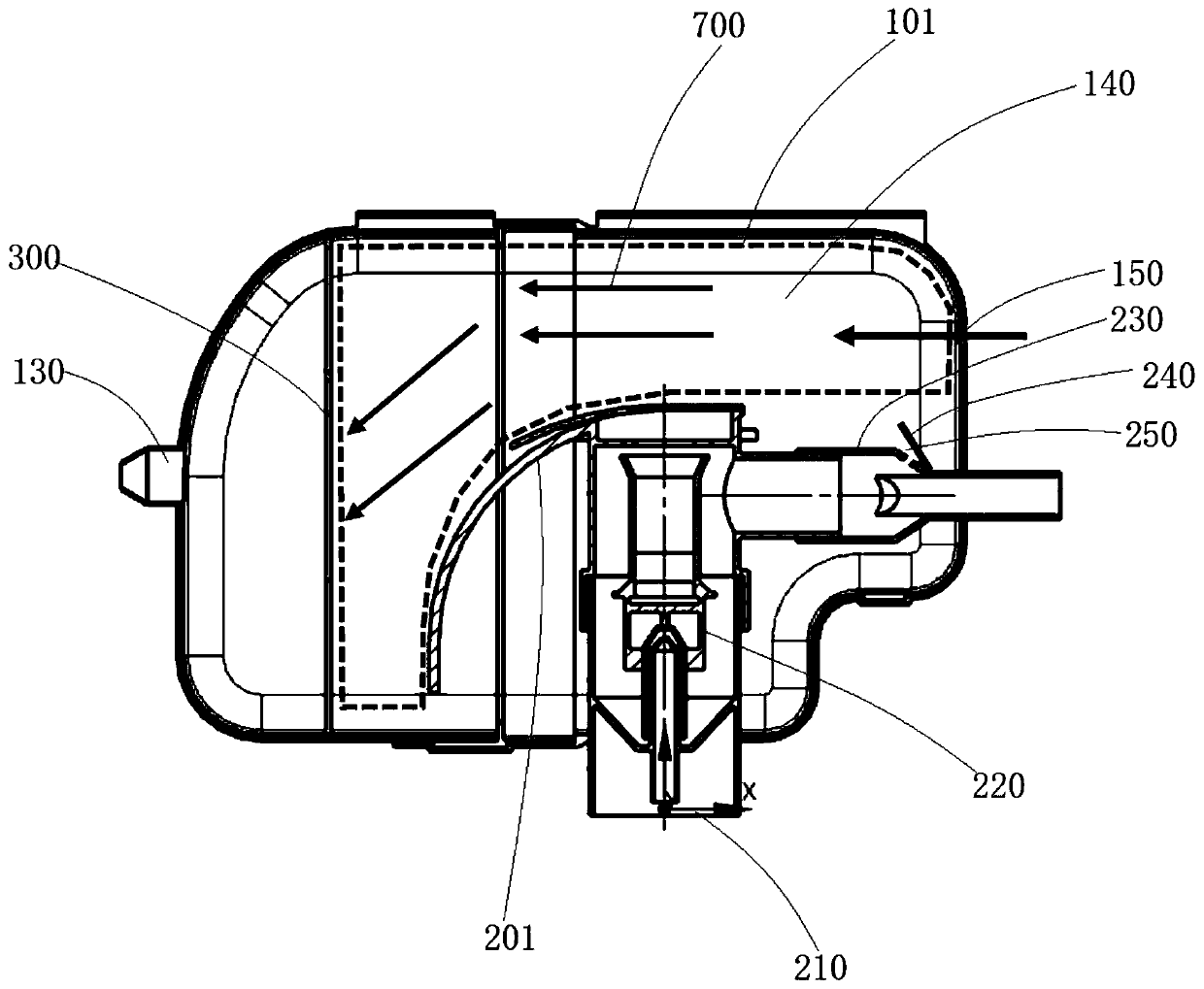
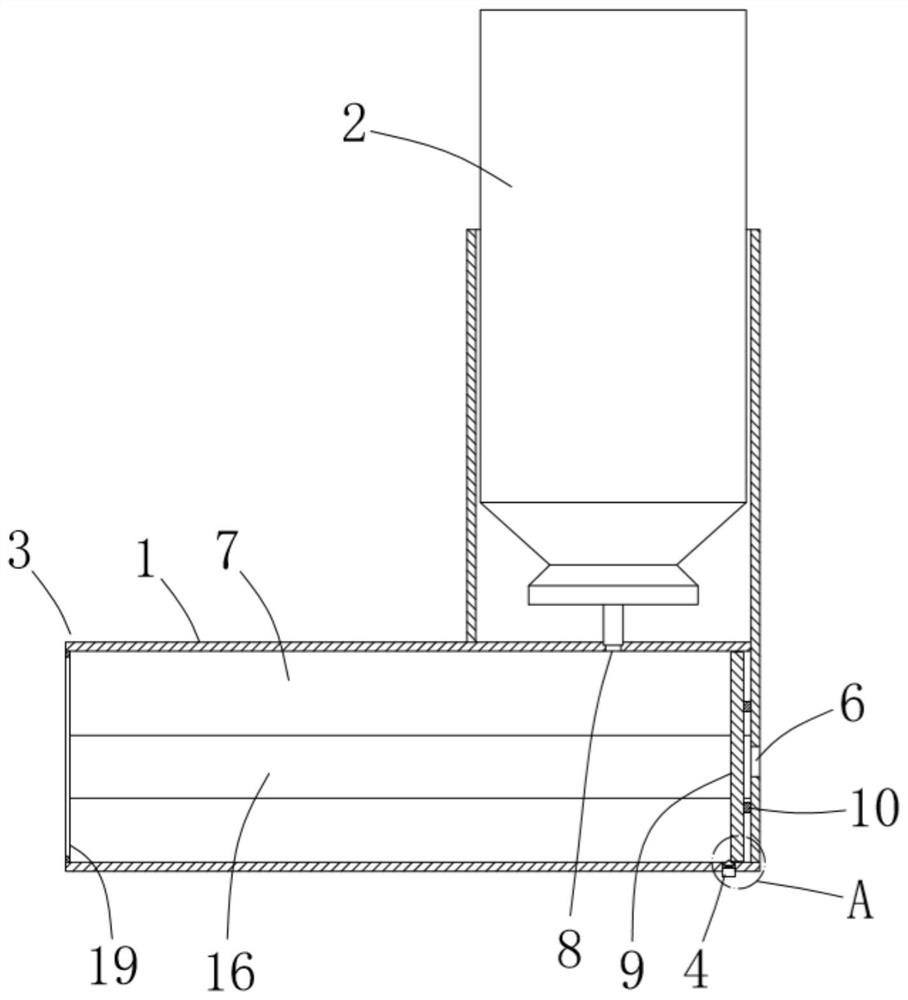
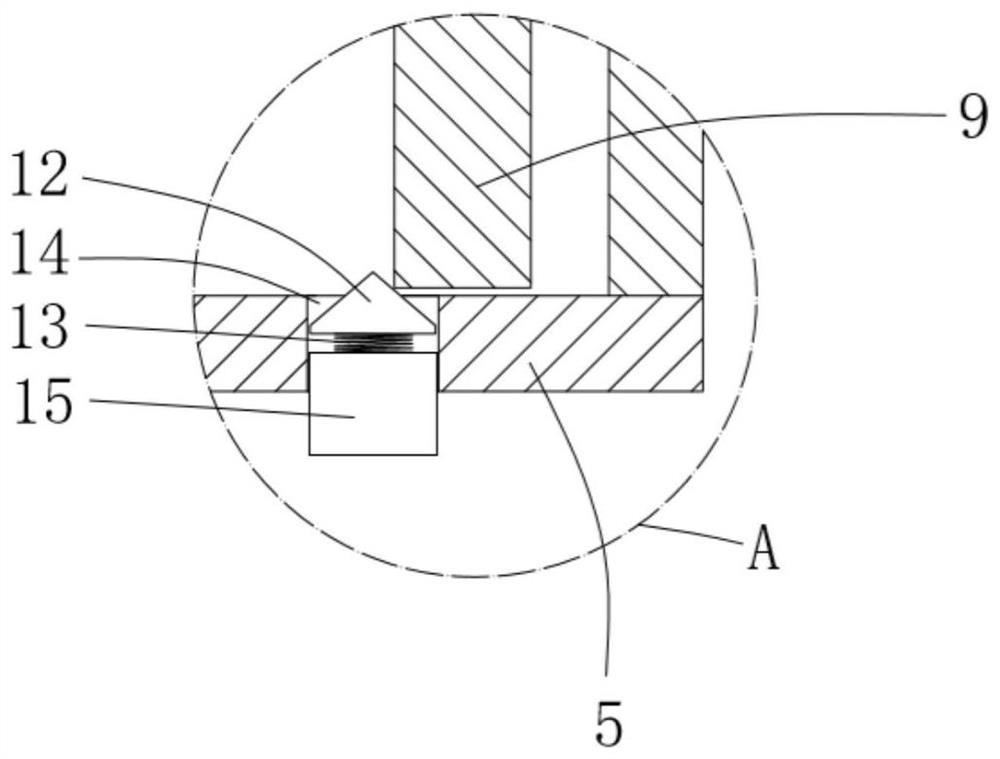
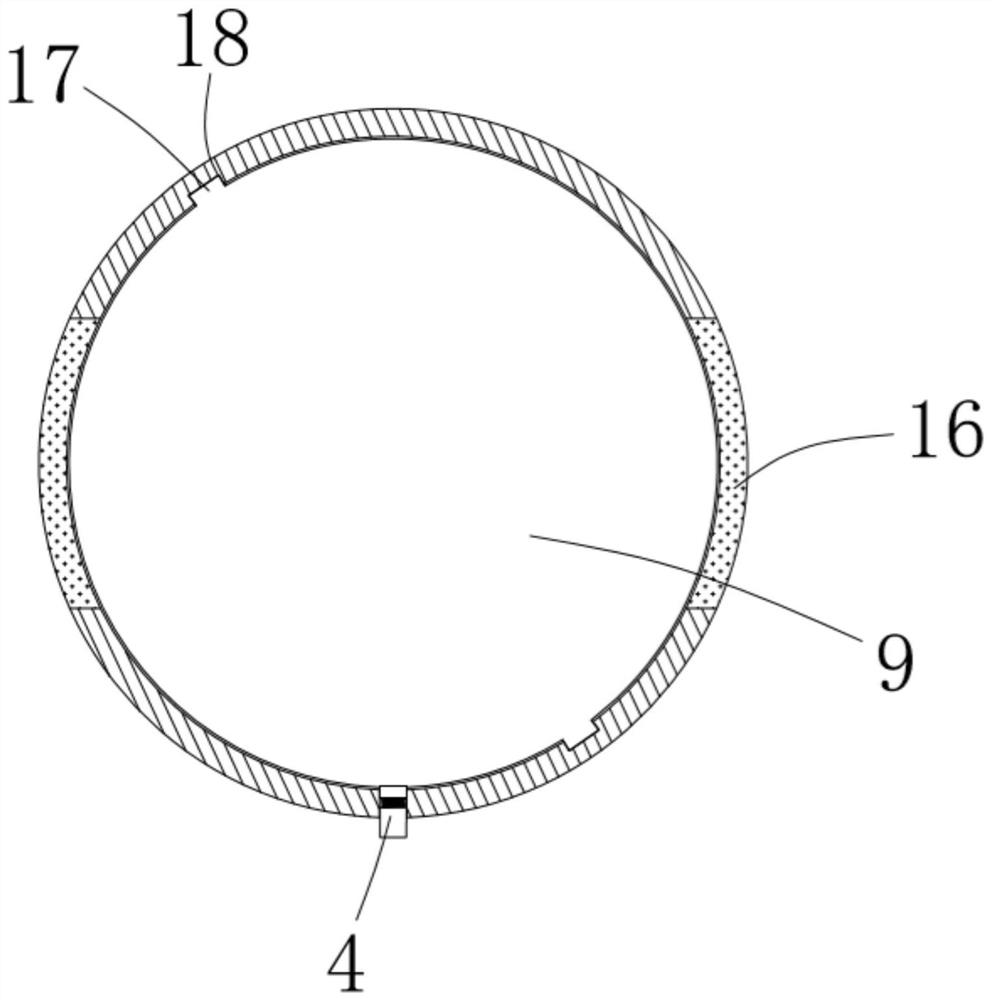
ActiveCN111956918AEliminate residueReduce the risk of adverse reactionsMedical atomisersInhalatorsUse medicationInhaled drug
The invention relates to the field of inhalant devices, in particular to an inhalant device favorable for inhalant to enter deep part of lung. The device comprises a shell, the shell is provided witha storage part used for containing a medicine storage tank and an inhalation part used for inhaling medicine by a patient. The inhalant device further comprises a threshold switch, the threshold switch has a braking state and an opening state, the threshold value of the threshold switch is matched with the value of negative pressure generated in the inhalation part when the patient inhales from the inhalation part, and when the threshold switch is in the braking state, the medicine is stopped, so that the patient cannot inhale the medicine through the inhalation part; and when the value of thenegative pressure generated when the patient inhales from the inhalation part is equal to or larger than the threshold value, the threshold value switch is changed from the braking state to the opening state and kept in the opening state, and the patient can inhale the medicine from the inhalation part. In the scheme of the invention, the medicine can enter the lung at a relatively high flow rate, so that a good medicine supply effect and a good treatment effect can be achieved, and the adverse reaction risk of medicament use is greatly reduced.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
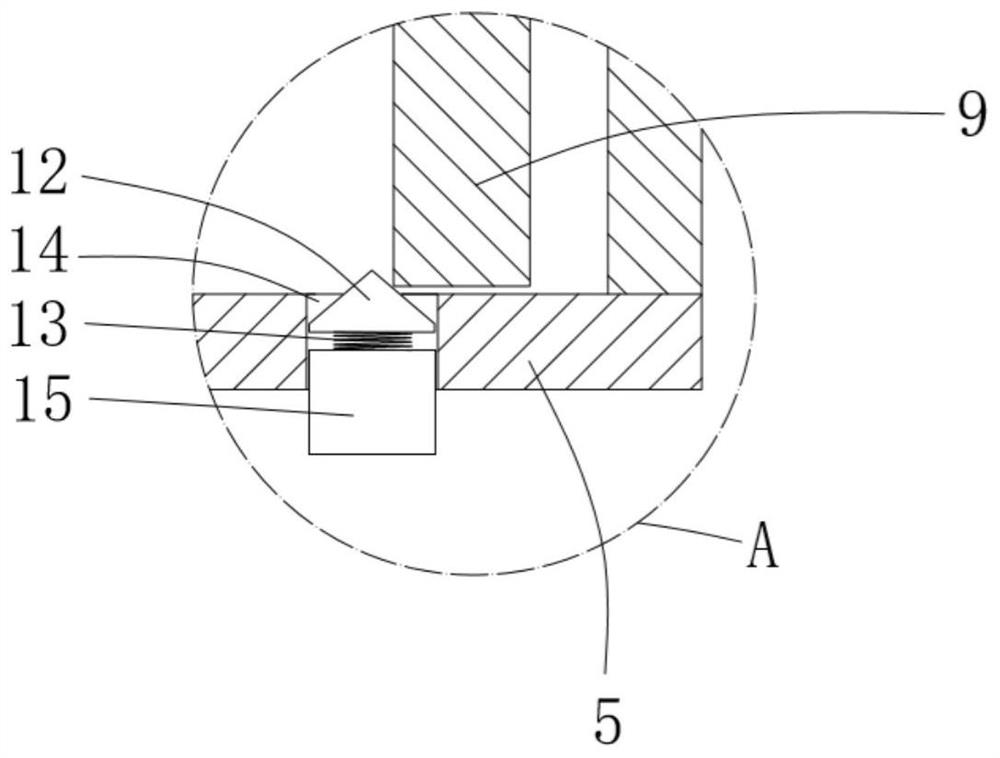
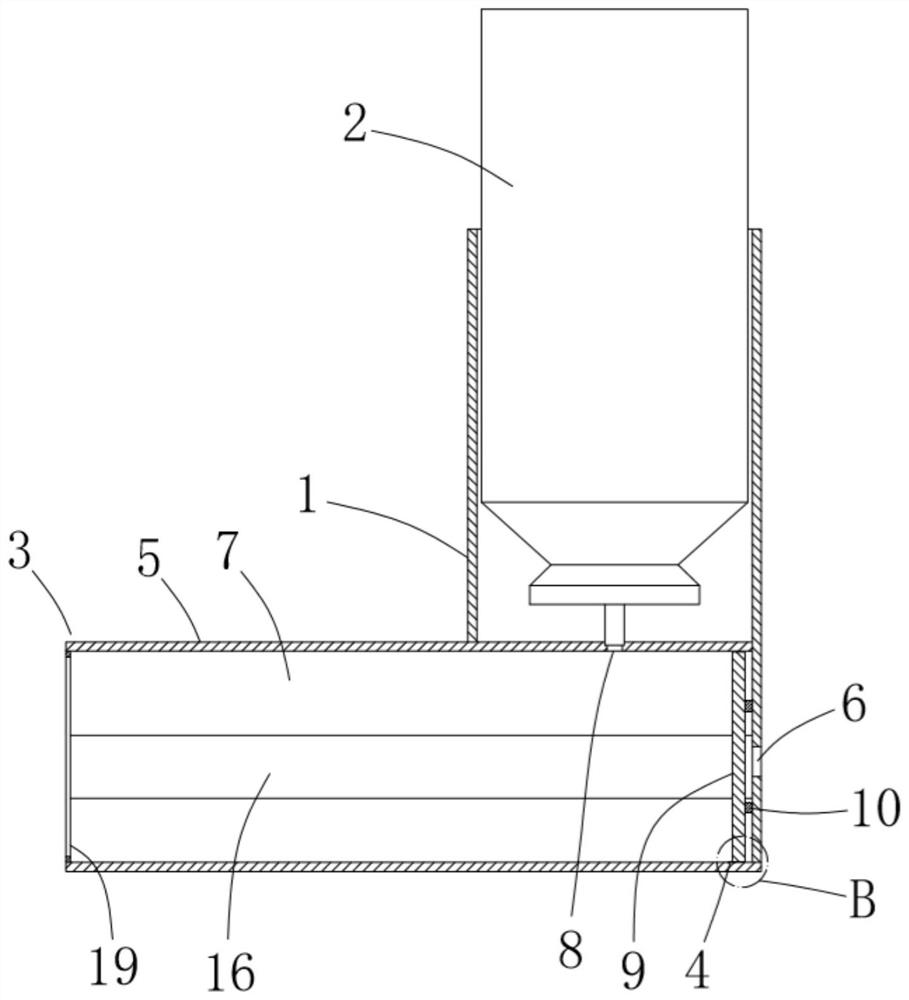
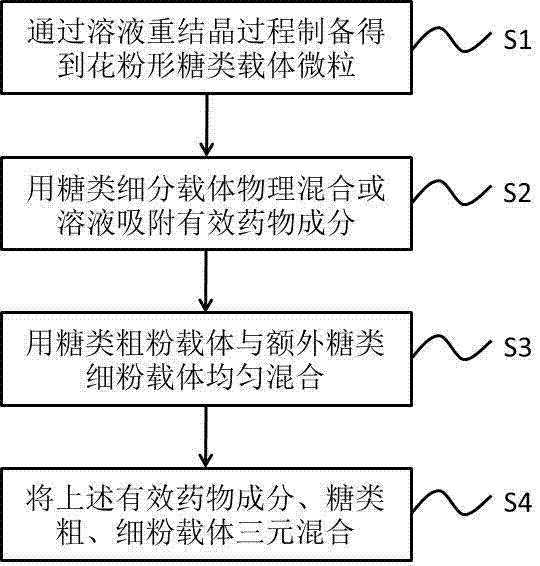
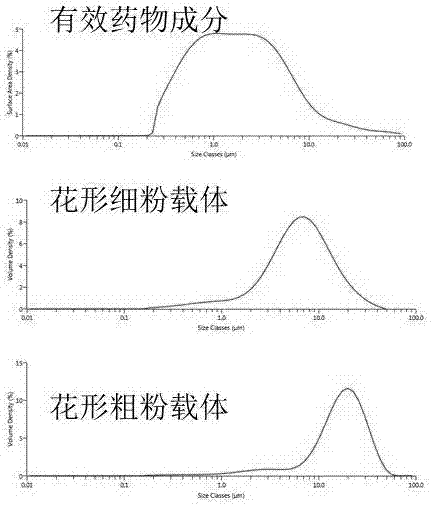
Dry powder inhalant matched with pollen-shaped saccharide carrier as well as preparation and application methods thereof
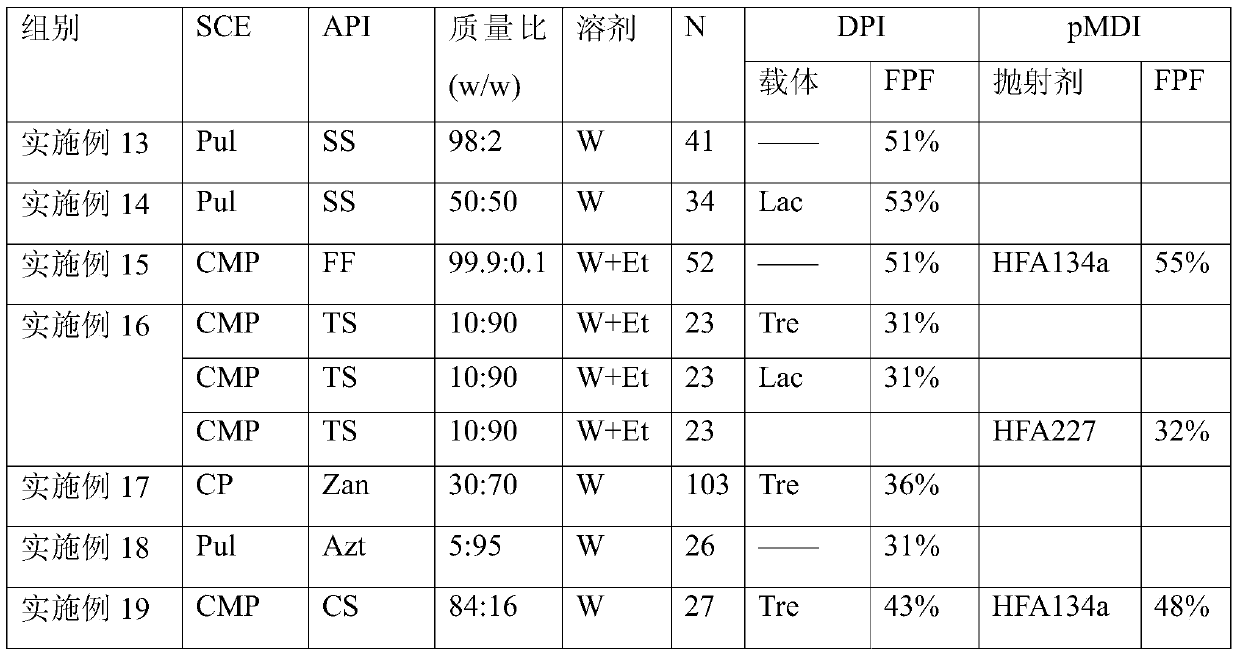
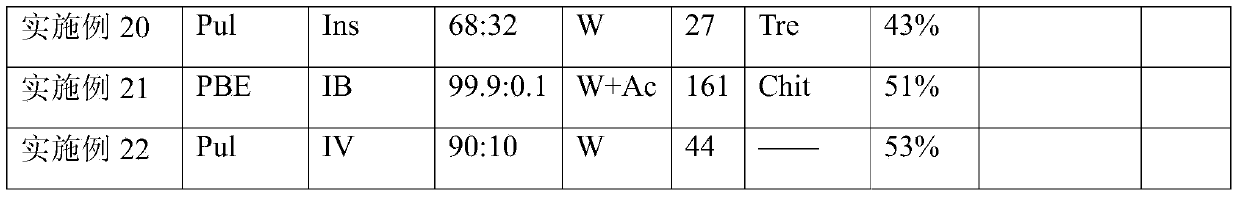
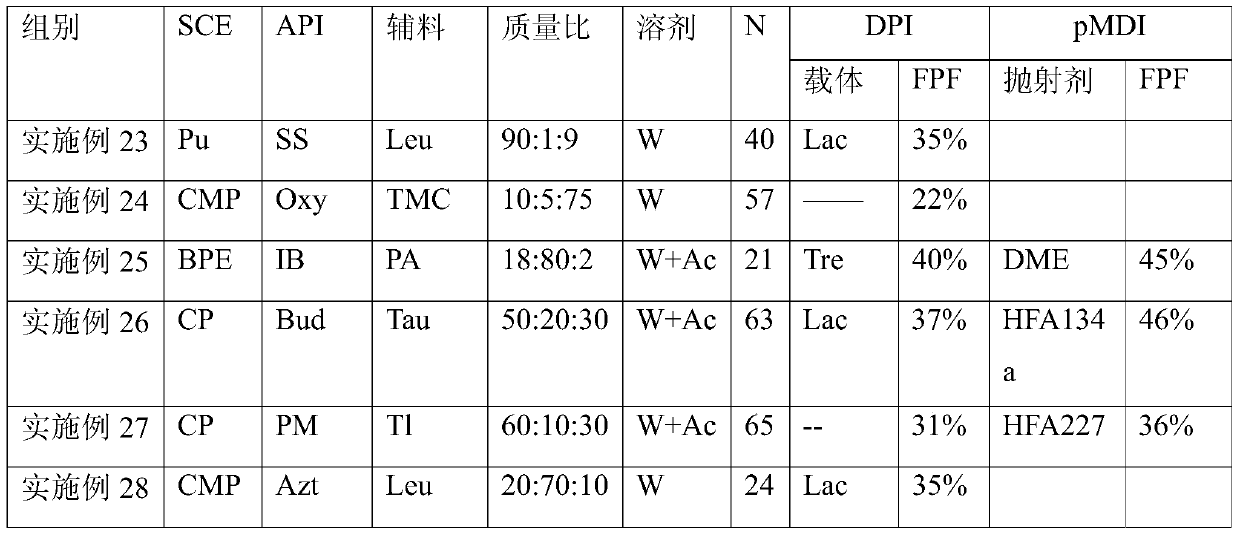
The invention provides a dry powder inhalant matched with a pollen-shaped saccharide carrier as well as preparation and application methods thereof. The dry powder inhalant comprises a ternary mixed component and the ternary mixed component comprises the following components: 0.01 to 2 parts of an effective medical component (with a preferred aerodynamic median diameter of 0.5 to 5 microns), 0 to 20 parts of a saccharide rough powder carrier (with a preferred aerodynamic median diameter of 10 to 100 microns) and 0 to 20 parts of a saccharide fine powder carrier (with a preferred aerodynamic median diameter of 1 to 10 microns), wherein the saccharide rough powder carrier and / or the saccharide fine powder carrier adopts prepared pollen-shaped saccharide carrier grains (the grains have rough surfaces and more pores inside and have a BET pore surface area of 10m<2> / g to 60m<2> / g; the density of the saccharide rough powder carrier is preferably 1 to 3 times as much as that of the saccharide fine powder carrier; the grain diameter of the saccharide rough powder carrier is preferably 5 to 10 times as much as that of the saccharide fine powder carrier; the fine powder distribution ratio (FPF) of the dry powder inhalant is 10%-50%. By adopting the preparation and application methods provided by the invention, higher drug loading amount, respiratory tract transportation capability and stability of inhaled drugs are realized and the dry powder inhalant has a better inhaled drug curative effect.
Owner:谭淞文
Inhalant device
The invention relates to the field of inhalant devices, and specifically relates to an inhalant device. The inhalant device comprises a shell, wherein a storage part for placing a drug storing pot andan inhalation part for a patient to inhale a medicament are arranged on the shell, and a medicament cavity communicating with the inhalation part is further formed in the shell and cooperates with the drug storing pot, and the medicament enters the medicament cavity when being sprayed from the drug storing pot; and a detection device for detecting the dose of the medicament in the medicament cavity is arranged on the inhalation part. The inhalant device provided by the invention can be used for monitoring the actual dose of the medicament inhaled by a patient, and when a detection result displays that the medicament is remained in the medicament cavity, the residual medicament can be inhaled by the patient again until being completely inhaled, so that the sufficient dose of the medicamentis guaranteed, and a good treatment effect is guaranteed.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Inhalant device with adjustable inhalation force
ActiveCN111905205AEliminate residueReduce the risk of adverse reactionsInhalatorsInhaled drugEngineering
The invention relates to the field of inhalant devices, in particular to an inhalant device with adjustable inhalation force. The device comprises an inhalation part for inhaling a medicament by a patient, and further comprises a threshold switch with a braking state and an opening state; a threshold of the threshold switch is matched with the value of negative pressure generated in the inhalationpart when the patient inhales air by the inhalation part, and when the threshold switch is in the braking state, the medicament is stopped, so that the patient cannot inhale the medicament by the inhalation part; when the value of the negative pressure generated when the patient inhales the air by the inhalation part is equal to or larger than the threshold, the threshold switch is converted to be in the opening state from the braking state and kept in the opening state, so that the patient can inhale the medicament by the inhalation part; and the threshold of the threshold switch is adjustable. According to the inhalant device, the good medicine supply effect and the good treatment effect can be achieved, the adverse reaction risk of medicine use is greatly reduced, and the inhalant device can be suitable for patients with different physical conditions.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Apparatus for simultaneously administering oxygen, and metered dose inhaler medication by inhalation
ActiveCN113950347ARapid responseGrow fastRespiratory masksMedical devicesInhaled drugPharmaceutical drug
An apparatus and method are provided for administering an inhaled drug to a person while simultaneously administering oxygen from a medical oxygen mask. The inhaled drug is from a pressurized metered dose inhaler (MDI), employing an extender tube about 3-10 cm long that fits into or over the mouthpiece of the inhaler. The MDI with extender tube is inserted into the mask and positioned so that the plume of drug travels through the extender when the MDI is actuated and is directed to just inside the mouth of the person. In an embodiment, an exhalation filter is provided to prevent contamination from infectious agents in the exhaled air from the person.
Owner:彼得·戈德曼 +1
Inhalation device capable of monitoring inhalation amount and used for inhalant device
ActiveCN111956919AActual intake monitoringRealize monitoringMedical devicesInhalatorsInhaled drugPhysical therapy
The invention relates to the field of inhalant devices, in particular to an inhalation device capable of monitoring the inhalation amount and used for an inhalant device. The inhalation device is usedfor being matched with the inhalant device for use. The inhalation device comprises a detection part for displaying the inhaled medicament amount of a patient; and the inhalation device is detachablyconnected with the inhalant device. According to the scheme, the detection part can be of a detection structure formed by combining an existing sensor capable of detecting the medicament amount and adisplayer, and can also be of a structure for displaying the inhaled medicament amount of the patient in other forms. The inhalation device is provided with the detection part for displaying the inhaled medicament amount of the patient, so that the actual inhaled amount of the patient is monitored.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Application of pullulan and its derivatives in the preparation of sustained and controlled release drug granules
ActiveCN105616390BProlong the action timeGood treatment effectOrganic active ingredientsPowder deliveryInhalable particlesControlled release
Owner:李浩莹
Inhalable medicament
InactiveUS20170027960A1Effective treatmentOrganic active ingredientsDispersion deliveryInhaled drugMedicine
The present invention provides an inhalable pharmaceutical solution aerosol comprising beclometasone dipropionate, ethanol and a propellant selected from 1,1,1,2-tetrafluoroethane, 1,1,1,2,3,3,3-heptafluoropropane and a mixture thereof, wherein the aerosol has a droplet size having a mass median aerodynamic diameter of 0.5-2.0 μm, for use in the treatment of asthma in an individual with impaired hand-inhalation coordination.
Owner:TEVA BRANDED PHARMA PROD R & D
An inhaler device that facilitates the inhalation of inhalants deep into the lungs
ActiveCN111956918BGood treatment effectReduce the risk of adverse reactionsMedical atomisersInhalatorsUse medicationInhaled drug
The present invention relates to the field of inhalant devices, in particular, to an inhalant device that facilitates inhalation of inhalants into the deep lungs, comprising a casing, on which is provided a storage portion for placing a medicine storage tank and a patient The inhalation part for inhaling the medicament, the inhalation device also includes a threshold switch, the threshold switch has a braking state and an open state, and the threshold value of the threshold switch matches the negative pressure value generated in the inhalation part when the patient inhales the inhalation part, when the threshold value switch When it is in the braking state, the medicine is blocked, so that the patient cannot inhale the medicine at the inhalation part; when the negative pressure value generated by the patient's inhalation at the inhalation part is equal to or greater than the threshold value, the threshold switch changes from the braking state to the open state, And keep the open state, so that the patient can inhale the medicine through the inhalation part. In the scheme of the present application, the medicament can enter the lungs at a relatively high flow rate, which can achieve a good drug supply effect and treatment effect, and greatly reduce the risk of adverse drug reactions.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
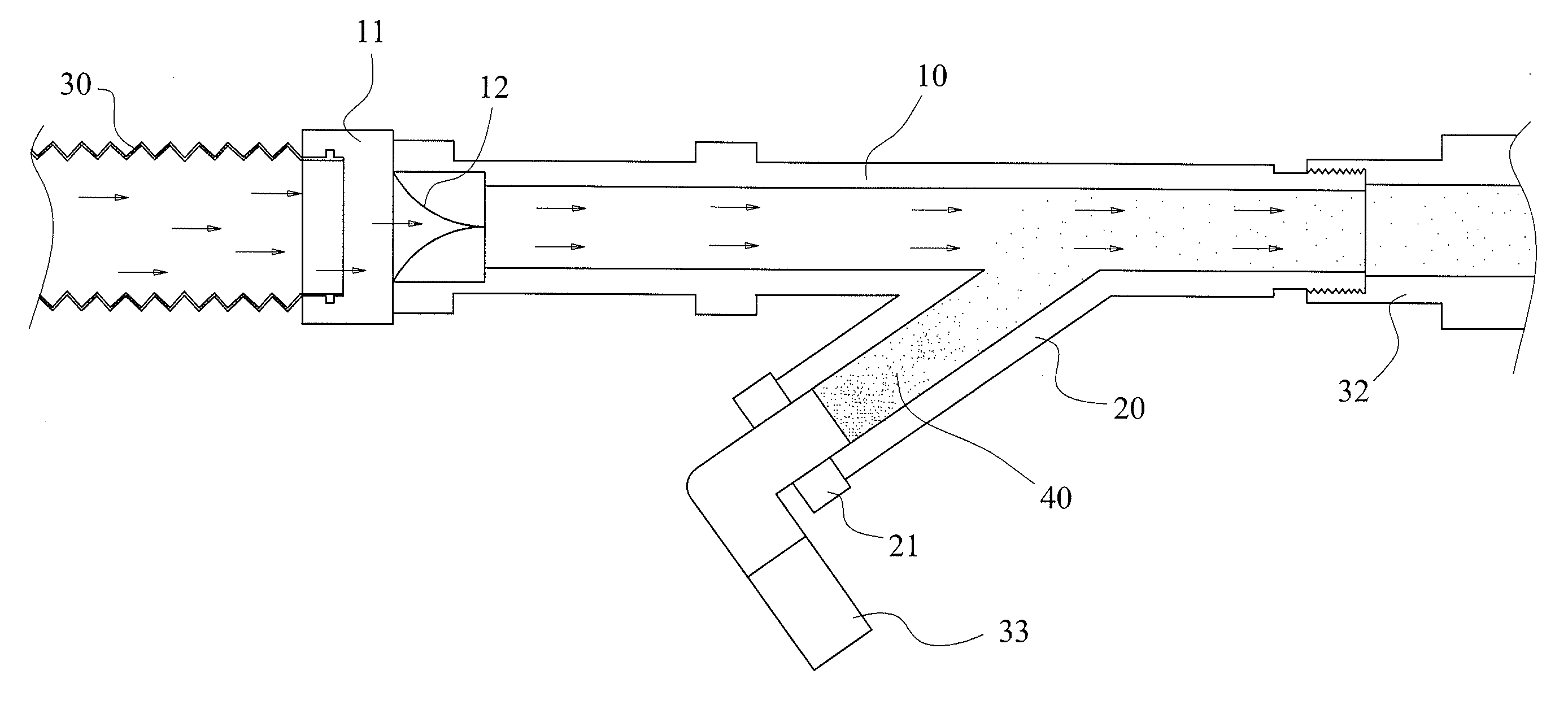
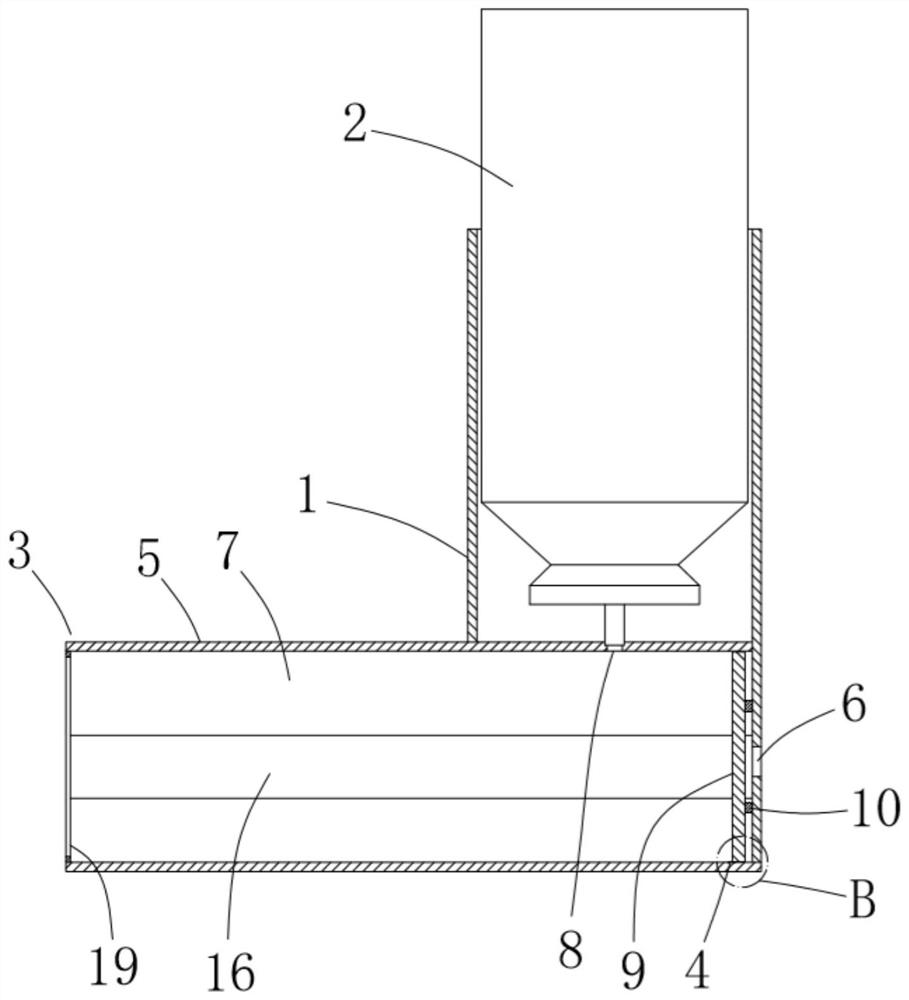
A drug delivery device with double nostrils for the respiratory tract
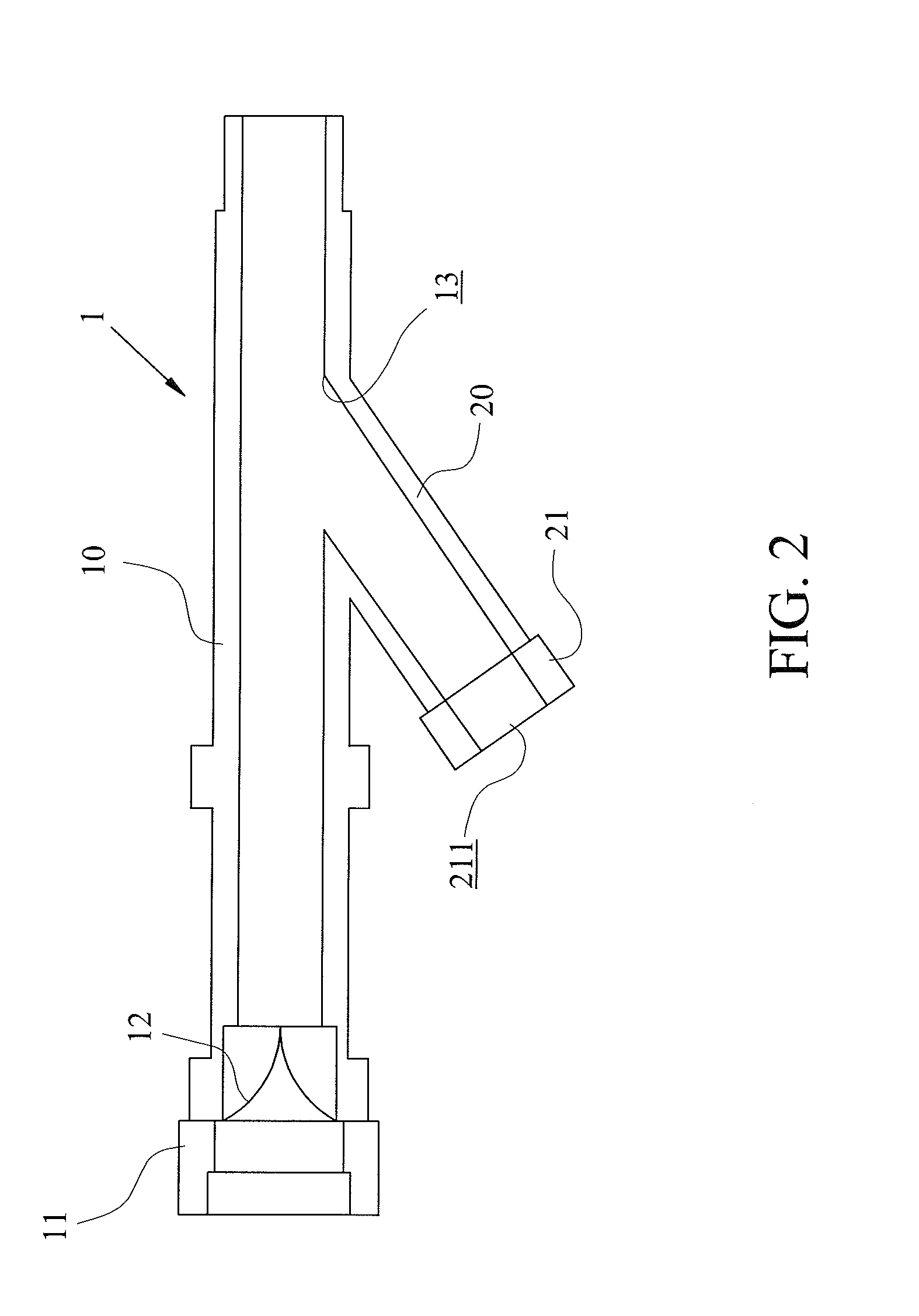
The invention relates to a drug inhaler and particularly relates to a respiratory tract dual-naris dosing device. An air inhaling and exhausting pipe (3) is respectively arranged on two pipe orifices of a T-branch pipe, the third pipe orifice of the T-branch pipe is provided with a connection pipe (6), the lower end of the connection pipe is connected with the inner wall of a fat pipe (10), the lower end of the connection pipe forms a sphere (8), the moving space of the sphere in the fat pipe on the upper portion of an inverted cone pipe (12) which is arranged at the lower end of the fat pipe, a slim pipe (13) is arranged at the lower end of the inverted cone pipe (12), an air inlet and outlet pipe (11) is arranged on one side of the fat pipe, the lower portion of the slim pipe is in inserted connection with the upper end of a drug inhaling pipe (14), and the sphere is located in the inverted cone pipe. According to the respiratory tract dual-naris dosing device, drug powders are distributed in two nares by using the T-branch pipe and the air inhaling and exhausting pipe, the drug powders synchronously enter focus parts in the two nares from the drug inhaling pipe, the fat pipe and the T-branch pipe when the sphere in the fat pipe and the inverted cone pipe controls inhaling drugs, and during expiration, the sphere closes the inverted cone pipe communicated with the drug inhaling pipe to achieve the purpose of saving the drug powders without affecting breathing.
Owner:张自鑫
Pharmaceutical administration to neonates, infants and children
PendingUS20220008666A1Effective treatmentOrganic active ingredientsDispersion deliverySmall airwaysDisease
The invention provides methods, devices and compositions for use in inhalation therapy of neonates, infants or children younger than 12 years, e.g. from 1 to 8 years, suffering from a disease, optionally a pulmonary disease, such as asthma, by which high amounts of inhaled drugs are directed to the small airways of the peripheral lungs of neonates, infants and children using slow, controlled flow rates and pre-set inhalation volumes. A novel inhalation device tailored for use in neonates, infants and children and adapted to provide said slow, controlled flow rates with a simplified jet nebuliser set-up is disclosed, together with kits comprising the device
Owner:VECTURA DELIVERY DEVICES
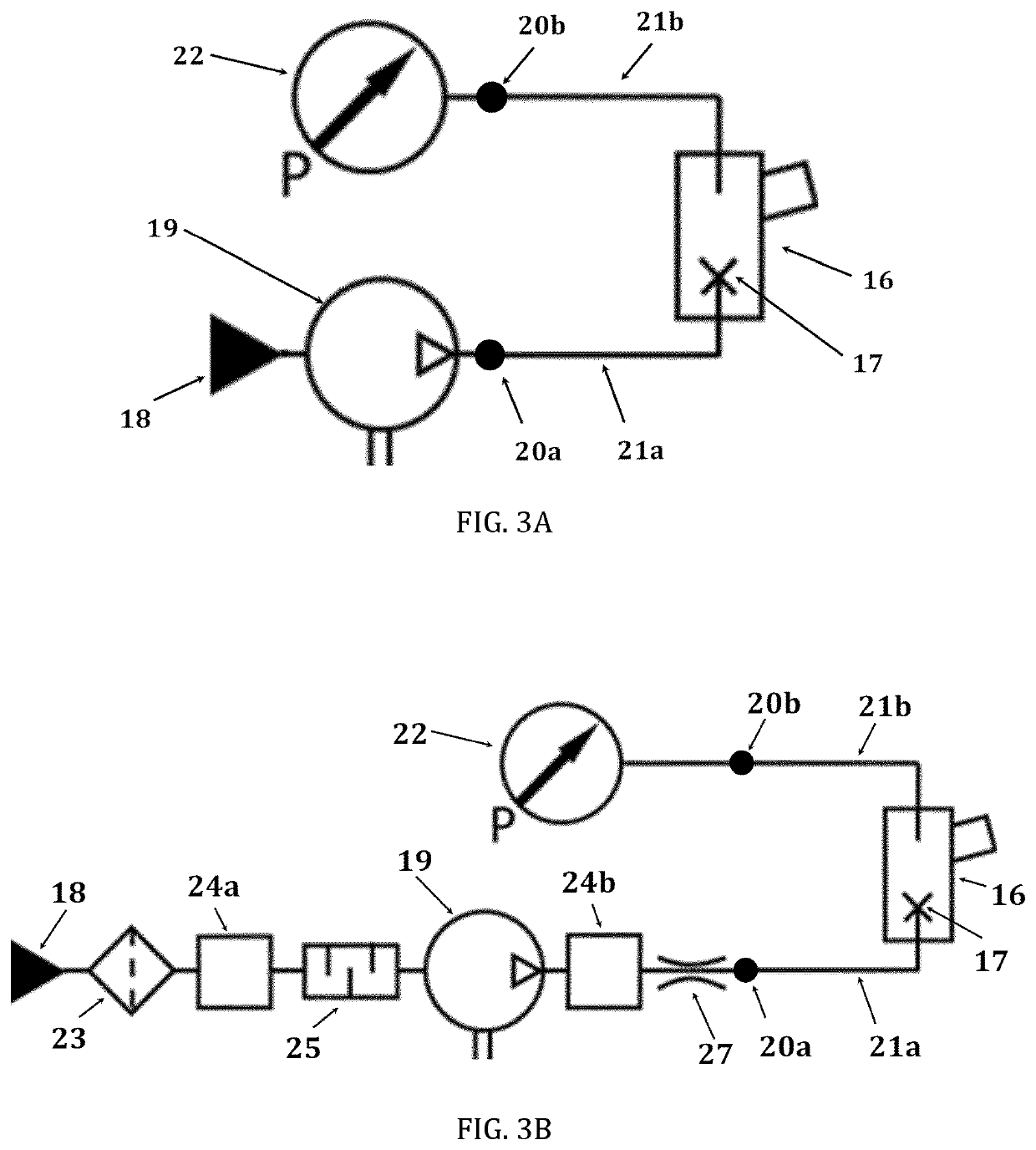
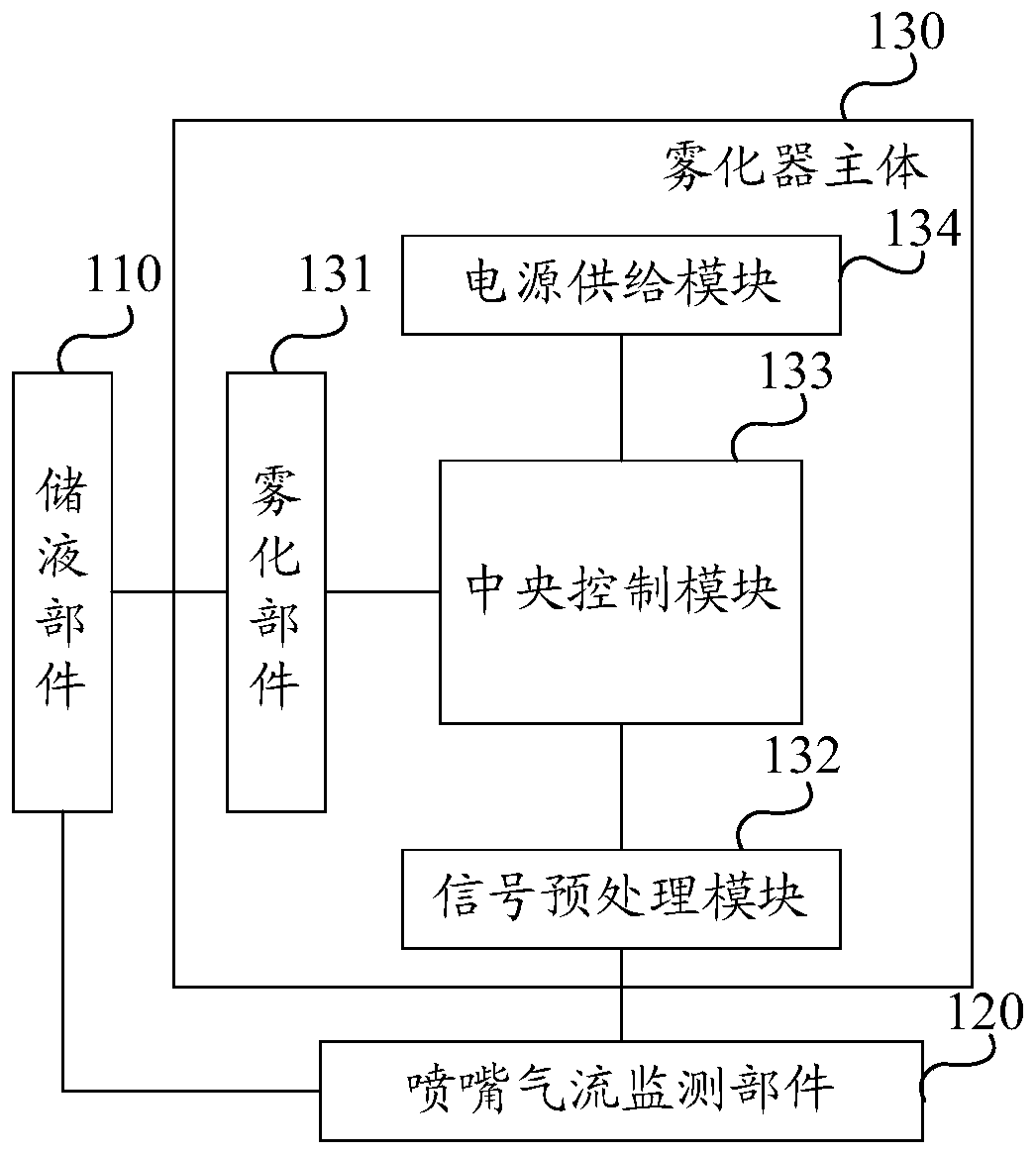
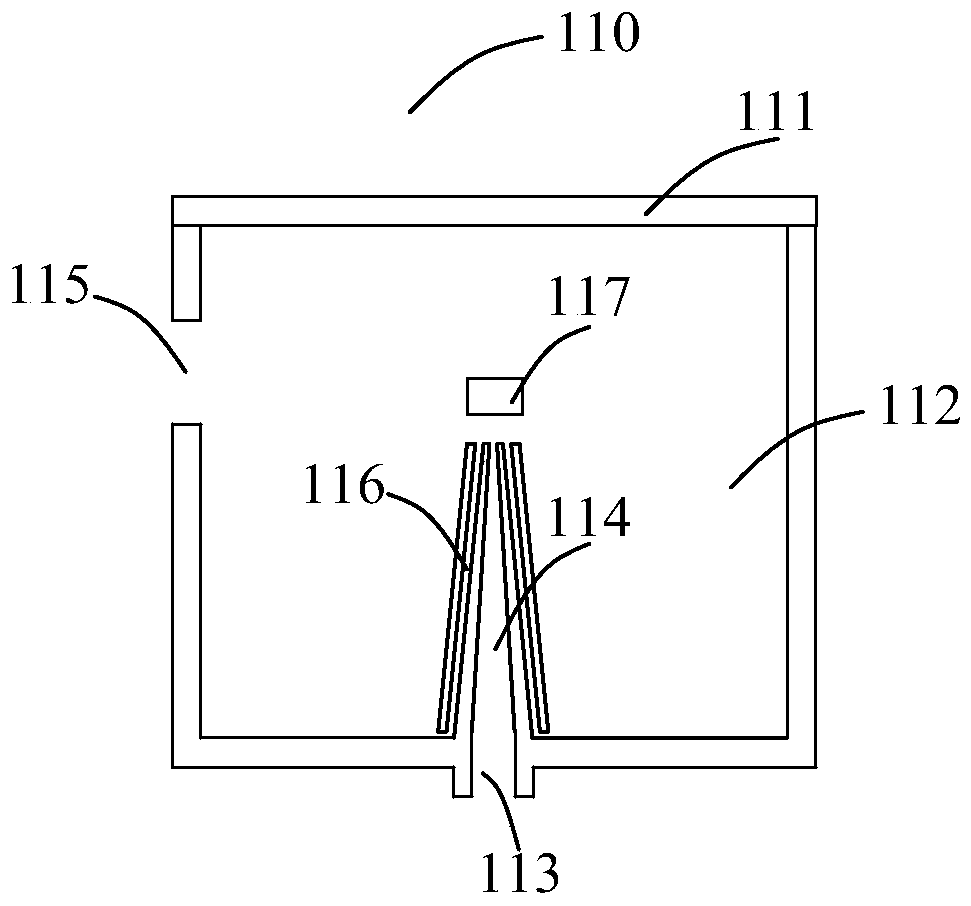
Nebulizer with drug intake monitoring function and drug intake monitoring system
ActiveCN108525082BMonitor inhaled dosesMonitor drug use timeMedical atomisersInhalatorsNebulizerInhaled drug
The invention discloses an atomizer and a drug absorption monitoring system with the function of monitoring the amount of drug absorption, wherein the atomizer comprises: a liquid storage part, a nozzle air flow monitoring part and an atomizer main body; wherein the liquid storage part , which is connected to the main body of the nebulizer, and is used to store the liquid medicine to be atomized and sprayed; the nozzle airflow monitoring part, which is connected to the liquid storage part, is used to output the airflow pressure electrical signal according to the airflow generated by the user's inhalation or exhalation, and The medicine liquid atomized by the nebulizer main body is sprayed into the mouth and nose of the user; the nebulizer main body is electrically connected with the nozzle air flow monitoring part, and is used for spraying the medicine liquid stored in the liquid storage part after nebulization, and According to the electrical signal of the airflow pressure output by the nozzle airflow monitoring component, analyze and calculate the amount of medicine inhaled by the user, and obtain the medicine inhalation information of the user. The nebulizer and the drug absorption monitoring system provided by the present invention can monitor the user's drug inhalation information sensitively and accurately.
Owner:NAZHIYUAN TECH TANGSHAN LLC
Isoflurane based tranquilizing drug
InactiveCN107397737ASedative/hypnotic fastFast fadingNervous disorderEther/acetal active ingredientsSide effectLiver and kidney
The invention relates to an isoflurane based tranquilizing drug. The isoflurane based tranquilizing drug includes a medicament and a drug carrier, wherein the medicament is isoflurane. The drug carrier comprises a water-absorbing material and a shell. The water-absorbing material is used for carrying isoflurane, and the shell is used for coating the water-absorbing material to avoid isoflurane volatilization. The shell is provided with an opening for volatilizing isoflurane at the opening in the treatment process, and the opening is provided with a sealing device. The isoflurane based tranquilizing drug provided by the invention is an inhaled drug accepted by breathing, has fast tranquilizing speed and small side effect, and the drug subsides quickly after taking effect, and does not affect the normal life and work of drug users. In addition, the isoflurane based tranquilizing drug has low bioconversion rate and minimal influence to liver and kidney functions, and is still suitable for patients with incomplete liver and kidney functions.
Owner:SICHUAN CANCER HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com